Setting Raw-Material Specifications Using Prediction Models: Determination of a Specification Limit for a Raw-Material Impurity in mPEG-AldehydeSetting Raw-Material Specifications Using Prediction Models: Determination of a Specification Limit for a Raw-Material Impurity in mPEG-Aldehyde
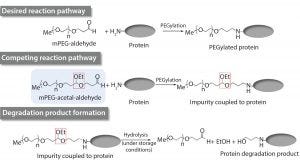
Figure 1: Proposed mechanism for the formation of the drug substance degradation product (2)
Impurities related to raw materials used for bioproduction can be inadvertently introduced into a manufacturing process, causing potential failure to meet in-process controls or release specifications. Unexpected impurities also can reduce yield and affect the quality, safety, and effectiveness of a final product (1). Raw-material impurities can originate from starting components or reagents used in manufacture. They can be generated in situ during synthesis or as degradation products. Impurities also can result from improper handling, packaging, and storage.
Identification and characterization of such impurities can provide insight into the mechanism of formation for raw material impurities as well as their potential impacts on final drug products (2). Such knowledge can be used further for the development of control strategies (e.g., incoming raw material release specifications) to ensure consistent process performance and product quality throughout a biological product lifecycle. Unfortunately, the effect of unexpected raw-material impurities upon a biological product is at times not fully realized until a major deviation occurs.
mPEG-aldehyde (monomethoxy polyethylene glycol aldehyde) has been used in the past few decades as a key raw material in manufacturing protein and peptide-based biological therapeutics (3). Modification of native proteins by coupling them with polyethylene glycol (PEGylation) can increase the half life of proteins in a patient’s bloodstream by decreasing the filtration rate while maintaining biological activity (4). Under storage conditions, however, those PEGylated proteins can form degradation products over time.
Zhang et al. showed that an impurity in the mPEG-aldehyde raw material used to manufacture a long-lasting PEGylated recombinant protein led to increased levels of a drug-substance degradation product under recommended storage conditions (2). The putative mechanism of formation of the drug-substance degradation product was defined by elucidating its chemical structure and subsequent analytical characterization (2). The authors reported that the mPEG-acetal-aldehyde (monomethoxy polyethylene glycol acetal aldehyde) impurity present in the mPEG-aldehyde raw material reacts with proteins to form a labile acetal link that can be cleaved hydrolytically under storage conditions, resulting in elevated levels of the protein degradation product (Figure 1) (2). As part of the investigation for this event, the supplier implemented corrective actions to control formation of this impurity. In addition, implementation of appropriate in-house controls to monitor this raw material impurity was necessary to ensure good quality of the drug substance.
Herein, we describe the strategy used to establish an incoming specification limit for levels of mPEG-acetal-aldehyde using a combination of analytical tools and small-scale models (SSM) of the commercial process. The reverse-phase high-performance liquid chromatography (RP-HPLC)–based specification is now used as part of incoming quality release of mPEG-aldehyde material before its use in PEGylated protein manufacture.
Materials and Methods
Para-Aminobenzoic Acid (PABA) Derivatization: We used an RP-HPLC methodology based on PABA derivatization (through reductive amination) as a basis for the development of the specification. We also used it to quantify the amount of mPEG-acetal-aldehyde in the raw material. That process converts the mPEG-acetal-aldehyde impurity into a small-molecule derivative (PABA-derivative), which is quantified using RP-HPLC with an ultraviolet (UV) absorbance detector using an Agilent 1100 system (Agilent Technologies).
For sample preparation, we mixed mPEG-aldehyde with PABA solution and a reducing agent directly in an HPLC vial. This led to reductive amination followed by acidic work-up to form the PEGylated PABA for analysis.
Accelerated Degradation Studies: We used recombinant protein and different lots of mPEG-aldehyde raw material to perform the PEGylation reactions. PEGylation reactions and their subsequent purifications took place at laboratory scale using a qualified SSM with established methods to derivatize the N-terminus of the recombinant protein.
We purified the PEGylation pools using ÄKTAexplorer 100 systems (GE Healthcare). Final purified material was aliquoted in 1-mL vials and incubated at 30° ± 1 °C for up to two months. Sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) and size-exclusion (SE)-HPLC analyzed the samples as described below.
We used SDS-PAGE to visually assess the levels of drug-substance degradation product in samples that were subjected to accelerated degradation studies. A reference standard of the un-PEGylated recombinant protein served as SDS-PAGE control because it had the same migration pattern as the degradation product. The total protein load was 10 µg, and we used a silver nitrate solution to stain the gels.
SE-HPLC analysis on the accelerated-degradation samples quantified the levels of drug-substance degradation product in the samples. We monitored protein elution using a UV absorbance detector (280 nm). The degradation product was detected easily because of the large difference in retention time relative to the main product.
Results
Detection of mPEG-Acetal-Aldehyde By PABA RP-HPLC: The first step in the control strategy design was development of an analytical method for detection and quantitation of mPEG-acetal-aldehyde impurity in the mPEG-aldehyde raw material. Derivatization of reactive aldehydes with 2,4-dinitrophenylhydrazine (DNPH) followed by HPLC analysis has been the most common approach for their quantification (5–7). This approach is suitable for simple sample matrices such as air and water, but it is not always practical because of limitations such as incomplete or reversible derivatization, poor stability, or lack of specificity.
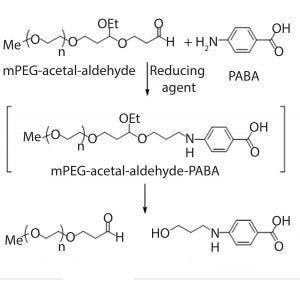
Figure 2: Derivatization of mPEG-acetalaldehyde impurity using PABA
Instead, we developed an RP-HPLC method based on reductive amination of aldehydes using 4-aminobenzoic acid (PABA) and a reducing agent to quantify mPEG-acetal-aldehyde impurity (Figure 2). PABA derivatization occurs before the RP-HPLC chromatography step. Because of the labile nature of the acetal linker (2), derivatization converts the polymeric mPEG-acetal-aldehyde into a small-molecule derivative, referred to as PABA-derivative. We quantified that derivative using RP-HPLC with an ultraviolet (UV) absorbance detector. This methodology allows for excellent separation and quantification (as PABA-derivative) of mPEG-acetal-aldehyde present in the mPEG-aldehyde. Quantification of the mPEG-acetal-aldehyde impurity enabled subsequent correlation of the synthesized structure to that of the observed drug-substance degradation product.
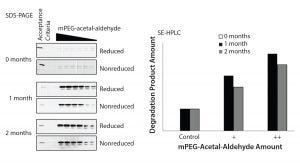
Figure 3: Formation of the drug substance degradation product reaches plateau after one-month under accelerated degradation conditions.
Establishment of Conditions for Accelerated Degradation Studies of Drug Substance Degradation Product: The next step in the design of the control strategy was to establish a reliable accelerated degradation methodology. Therefore, it was necessary to establish the minimum incubation time needed to break the acetal link leading to the formation of the drug-substance degradation product. To determine that time, we subjected SSM drug-substance samples to accelerated degradation conditions of 30° ± 1 °C for 0, 1, and 2 months. The smaller drug-substance degradation product has a significantly different molecular weight relative to the PEGylated protein drug substance (Figure 1). Therefore, we could detect and monitor it with SE-HPLC and SDS-PAGE (Figure 3).
Results show that the drug-substance degradation product reaches a plateau after one month under selected conditions. Therefore, we chose a one-month incubation time for all studies. Understanding the maximum amount of degradation product formed during development studies was critical to establishing a prediction model for controlling the impurity at the raw-material level.
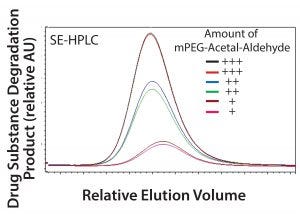
Figure 4: Drug substance degradation product levels correlate with mPEG-acetal-aldehyde impurity in the mPEG-aldehyde raw material.
Correlation Between mPEG-Acetal-Aldehyde and Drug Substance Degradation Product: After successful development of the PABA RP-HPLC method and an accelerated degradation model, the next step in developing the control strategy involved correlation between the raw material impurity and the drug-substance degradation product. First, we detected the levels of mPEG-acetal-aldehyde present in multiple lots of mPEG-aldehyde using the PABA RP-HPLC method. With this information, we selected mPEG-aldehyde lots with a wide range of mPEG-acetal-aldehyde levels to generate drug substance using the qualified SSM. We placed the resulting SSM drug-substance samples under accelerated degradation conditions to force cleavage of the mPEG-acetal-aldehyde labile link leading to the formation of the degradation product. Samples were then analyzed by SE-HPLC. Figure 4 shows the levels of degradation product of the PEGylated recombinant protein correlated with the levels of the mPEG-acetal-aldehyde impurity present in the mPEG-aldehyde.
As a next step, we used an orthogonal approach to confirm correlation between mPEG-acetal-aldehyde and the drug substance-degradation product. We generated new SSM samples using a single mPEG-aldehyde lot spiked with increasing amounts of in-house synthesized mPEG-acetal-aldehyde. We also subjected samples generated with spiked mPEG-aldehyde to accelerated degradation conditions.
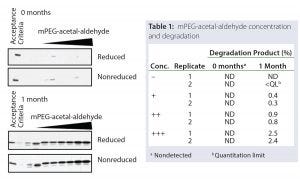
Figure 5: Spiking studies show correlation of mPEG-acetal-aldehyde amount with degradation product levels.
SDS-SAGE and SE-HPLC analyses monitored drug-substance degradation. Degradation product was detected only after long-term storage and under accelerated degradation conditions. Thus, as expected at storage time zero, levels of the degradation product were within the SDS-PAGE acceptance criteria and not detected by SE-HPLC (Figure 5).
However, both SDS-PAGE and SE-HPLC analyses detected an increase in drug-substance degradation product after one month under accelerated degradation conditions (Figure 5). More important, the increase in drug-substance degradation product was proportional to the amount of mPEG-acetal-aldehyde spiked into the material. Our experiments confirmed that the levels of drug-substance degradation product were directly correlated to the amount of mPEG-acetal-impurity in the mPEG-aldehyde raw material.
Development of a Prediction Model and Raw-Material Specification: With a correlation established between levels of mPEG-acetal-aldehyde and the drug-substance degradation product, we generated a statistical model to predict the formation of degradation product during drug substance long-term storage. We used the model with an understanding of process clearance capability to support setting an appropriate specification limit for incoming mPEG-aldehyde. To generate the prediction model, we evaluated 10 mPEG-aldehyde lots with known and increasing concentrations of the mPEG-acetal-aldehyde impurity.
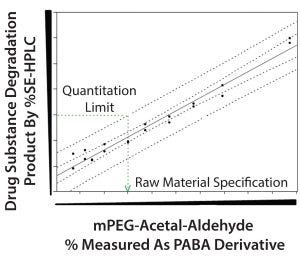
Figure 6: Prediction model to establish mPEG-acetal-aldehyde specification limit
Samples generated using SSMs were subjected to accelerated degradation conditions to detect formation of drug-substance degradation product. We incubated samples for one month and then used SDS-PAGE and SE-HPLC analysis. Figures 6 and 7 show SE-HPLC degradation product levels plotted against known levels of mPEG-acetal-aldehyde in the selected mPEG-aldehyde lots. A linear correlation can be seen between the levels of mPEG-acetal-aldehyde and the drug substance degradation product. Linear regression analysis showed a good fit with R2 = 0.97.
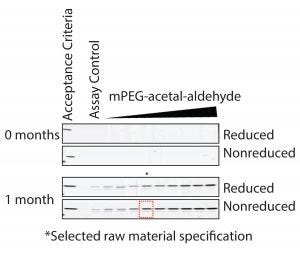
Figure 7: Formation of degradation product under accelerated degradation conditions
To unambiguously establish the mPEG-acetal-aldehyde specification limit, we determined individual predicted values (99% predition intervals) and overall expected values (95% confidence intervals). We evaluated mPEG-acetal-aldehyde specification limit based on PABA RP-HPLC against the drug-substance degradation product upper prediction interval corresponding to the quantitation limit (QL) of the SE-HPLC analytical method (Figure 6). The mPEG-acetal-aldehyde value that corresponded to the SE-HPLC method QL passed the SDS-PAGE acceptance criteria for drug-substance degradation product (Figure 7). The specification limit for the mPEG-acetal-aldehyde in the mPEG-aldehdye raw material will ensure that levels of the degradation product (un-PEGylated protein adduct) remain within acceptable levels during drug-substance storage.
Successful Raw-Material Control
Appropriate control of previously unknown impurities in raw materials used in manufacturing biological products is critical to ensure product quality and patient safety. Such controls also play an important role in reducing operational risk in manufacturing plants. In this case study, we describe the method used to create a specification limit for an impurity discovered in the raw material used for synthesis of a PEGylated recombinant protein. We used small-scale models and accelerated degradation studies and developed an analytical method for detection of the impurity in the raw material to develop a successful control strategy. Correlations established between degradation rate of the PEGylated recombinant protein and the levels of mPEG-acetal-aldehyde present enabled final development of the specification limit based on PABA RP-HPLC results.
Currently, the established specification limit serves as an important incoming control for all mPEG-aldehyde lots to ensure high quality of the product through its lifecycle.
Acknowledgment
The authors thank the chemical process research department of Amgen Inc., (Thousand Oaks, CA) for providing the material for the mPEG-acetal-aldehyde spiking studies.
References
1 Cordoba-Rodriguez R. Raw Materials in the Manufacture of Biotechnology Products: Regulatory Considerations. PDA J. Pharm. Sci. Technol. 64(5) 2010: 445–450.
2 Zhang B, et al. Analytical Characterization of a Novel Degradation Product in a PEGylated Recombinant Protein. J. Pharm. Sci. 100(11) 2011: 4607–4616.
3 Kling J. PEGylation of Biologics a Multipurpose Solution. BioProcess Int. 11(3) 2013: 34–43.
4 Seely JE, et al. Making Site-Specific PEGylation Work. BioPharm Int. 18(3) 2005.
5 Casella IG and Contursi M. Determination of Aliphatic Aldehydes By Liquid Chromatography with Pulsed Amperometric Detection. J. Chromatog. A 1063, 205: 129–135.
6 Isakau H, Robert M, and Shingel KI. A Novel Derivatization-Free Method of Formaldehyde and Propylene Glycol Determination in Hydrogels by Liquid Chromatography with Refractometric Detection. J. Pharma. Biomed. Anal. 49, 2009: 594–600.
7 Uchiyama S, Inaba Y, and Kunugita N. Determination of Acrolein and Other Carbonyls in Cigarette Smoke Using Coupled Silica Cartridges Impregnated with Hydroquinone and 2,4-Dinitrophenyl-Hydrazine. J. Chromatog. A 1217, 2010: 4383–4388.
Corresponding author Lisandra Negron-Vega, PhD, is a senior scientist, Kirla Mauras is a scientist, Arturo Hornedo is a senior validation scientist, and Alejandro Toro is a director, all in process development at Amgen Manufacturing Ltd., PO Box 4060, RD 31 Km 24.6, Juncos, PR 00777; [email protected]. Belle Liu is a quality assurance specialist in clinical plant at Amgen Inc., One Amgen Center Drive, Thousand Oaks, CA, 91320-1799.
You May Also Like






