WWW.COPPERHOUNDPICTURES.COM
It surprises some folks in the BPI community to learn that I’m a journalist by training, boasting a mere bachelor of arts degree in journalism despite my high-fallutin’ technical editor title. I was originally trained in Associated Press (AP) style by a veteran newsman who spent many years working for AP’s main competitor, United Press International (UPI). When I first started on
BioPharm
back in 1996, I had to get up to speed quickly on the stylebooks of Chicago and the American Chemical Society — more commonly used in the magazine and scientific journal worlds — and I never lost my journalistic sensibilities. So I was happy to manage the news-briefs sections of both magazines I’ve worked on, first as
BioPharm’s
assistant editor, then associate and managing editor, and finally now as BPI’s senior technical editor.
A monthly magazine must take a different approach to news from that of a daily paper or weekly newsletter. Timeliness is not an option. We’ve always considered o...
Holding True to the Price-Cap Pledge
As in previous years, 2018 started with more than 30 drugs increasing in price on the US market. However, data and analysis firm GlobalData observes that criticism from politicians and the public seems to be keeping the industry to a 10% self-imposed price-hike limit. It began with Allergan CEO Brent Saunders pledging to police pricing in 2016, when he stated that his company would limit itself to one price increase per year and only by single-digit percentages. And none of the price hikes seen so far this year have exceeded 9.9%. Companies such as Teva, Cellectis, Insys, Synergy, and Supernus all have raised prices while staying within the 10% limit. Allergan meanwhile has raised the price of 18 medications by 9.5%.
GlobalData healthcare analyst Alice Stevens says, “As rising drug prices continue to be a major cause for discord in the United States, self-regulation of price hikes could help the industry prevent regulatory reform.”
This conservative approach to price i...
Panelists (from left to right) Ruby Casareno, Yuyi Shen, Xiao-Ping Dai, Tad Thomas, and Anne Montgomery
On 21 March 2018, a panel discussion during BPI West in San Francisco addressed ways to improve working relationships between sponsors and contract manufacturing organizations (CMOs). Our goal as organizers was to avoid retreading overfamiliar ground: Many outsourcing articles stress the importance of good communication without delving into actual strategies for improvement. So I asked the panelists to focus on the word
better
and what that actually means in this context.
The most productive way to approach such a discussion was to identify from examples where and how team expectations can become misaligned from the start of a project. That helped the panel focus on specific strategies they have found for creating better working relationships.
Because the panel did not include representatives from CMOs or contract development and manufacturing organizations (CDMOs), we began by encouraging those perso...
ADOBE STOCK (HTTPS://STOCK.ADOBE.COM)
Analytical method transfers are essential components of the current global biotechnology environment.
Analytical method transfer
can be defined as “a documented process that qualifies a laboratory (the receiving laboratory) to use a validated analytical test procedure that originated in another laboratory (sending laboratory), thus ensuring that the receiving laboratory has the procedural knowledge and ability to perform the transferred analytical procedure as intended” (
1
). The goal is to ensure that a method continues to perform in the validated state regardless of a change in the testing laboratory. Transfers can occur between laboratories at the same site, between sites at the same company, or between companies and/or regulatory laboratories. The requirements for such transfers change throughout the lifecycle of a method.
That background set the stage for the CASSS CMC Strategy Forum “Methods on the Move: Addressing Method Transfer Challenges for the Biopharma...
ADOBE STOCK (HTTPS://STOCK.ADOBE.COM
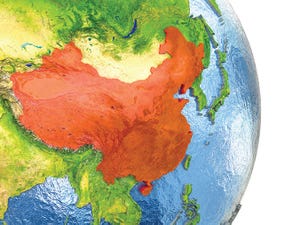
Over the past year, most of China’s biomanufacturing facilities have been engaged in active facility expansion. Based on our research of facilities under active construction, that growth has expanded total capacity in China by over 10%. Our research updates the Top 60 Biopharmaceutical Manufacturers in China directory from BioPlan Associates (
1
) and shows a continuing increase in new bioprocess capacity. This trend is unlikely to abate. As well as contacting our top 60 biomanufacturers, in fact, we contacted a number of smaller companies and industry observers. The resulting data show that a majority of Chinese companies — including makers of therapeutic monoclonal antibodies (MAbs), recombinant insulins, and vaccines, as well as contract manufacturers of biologics — expanded/upgraded their facilities in 2017.
For example, Shanghai Henglius Biotech (a leader in MAb development in China) expanded its bioreactor capacity by four 2,000-L units in 2017. The same company ...
STORYBLOCKS (WWW.STORYBLOCKS.COM)
Even with increased understanding of host cell proteins (HCPs) and their potential risks, no practical approach has been made available for HCP risk management during bioprocess development. A BioPhorum Development Group (BPDG) team has identified common HCP-related risk factors and built a template for semiquantitative risk assessment during process development based on publicly available information. To this end, the BPDG HCP working team’s assay and knowledge-sharing experts have established a common HCP risk assessment tool and mitigation strategy to guide bioprocess developers.
In part 1
last month, we introduced this tool and described how to apply it when high levels of residual HCPs are found in a drug substance (DS). This month, we examine several other applications as well as address the limitations of our risk-assessment tool.
More Potential Negative Events
Dilutional Nonlinearity Issues with Sample Testing:
In measurements of residual HCP in drug substance ...
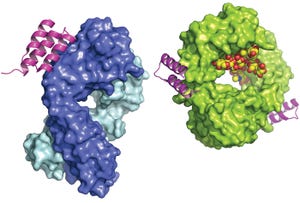
Protein A domain as a three-helix bundle binding to the heavy variable chain of a human Fab (left); minimized protein A bound to an Fc fragment (right). WIKIMEDIA COMMONS (WWW.WIKIPEDIA.COM)
Protein A affinity chromatography is a well-established technology that is used extensively for large-scale purification of monoclonal antibodies (MAbs). With this mode of chromatography, very high product purity can be achieved in a single, relatively simple unit operation. A solution containing the target protein of interest is applied to a liquid-chromatography column at near-neutral pH, and one or more wash steps follow to lower product- and process-related impurities (
1
). Product is eluted through application of a low-pH buffer. Finally, the resin is regenerated and prepared for use in a subsequent purification cycle.
Because of the powerful purification performance and relatively simple operation of this chromatographic step, protein A affinity resins are the foundation of MAb purification platforms (
2
). At ...
ADOBE STOCK (WWW.STOCK.ADOBE.COM)
The practice of quality control is promoted by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA). A number of process control charts are used in many biopharmaceutical companies to increase production and lower process costs. Here we focus on performance of three commonly used control charts: Shewhart control charts with WECO and supplementary rules, monitoring charts with exponentially weighted moving average (EWMA), and cumulative sum (CUSUM) charts.
WECO rules got the name from a quality control handbook published by the Western Electric Company in 1956 (
1
). Those rules and the additional four supplementary rules are used with Shewhart control charts in statistical process control (SPC) for detecting out-of-control signals before control limits are exceeded. For simplicity, we refer to all eight rules as
WECO rules
. In the handbook, the authors extensively discuss the range
of tests to detect abnormal patterns. The theoretical probabilit...
CPhI Worldwide (organized by UBM) announced last fall the final section findings of the fifth edition of the
CPhI Annual Report
. Presented live at the meeting in Frankfurt, Germany, the report is now available online. It highlights immediate and long-term trends in pharmaceutical data, regulation, generics, and biosimilars. Four experts gave their views.
They warned that the US Food and Drug Administration’s (FDA) approach to achieving six-sigma (nearly perfect or 99.9997% defect-free) quality is failing in respect to the pharmaceutical industry’s self-adoption of continuous quality systems, making regulatory oversight imperative. One positive note was identified in the current regulatory approach to data standards and analysis. It could lead, for example, to investments in handling “big data” that could revolutionize drug discovery and patient treatment over the coming decade.
A Contentious Relationship
The report also notes that, despite what one expert described as “abusive and unethical antics” of i...