BPI’s June issue always precedes a break in our regular issue schedules in July and August. But summer does not afford us any leisure. This July, we again shall bring you an eBook of summaries from the BPI Theater at the Biotechnology Innovation Organization’s annual convention (this year from digital presentations, which will be available in full on the BPI site) along with our annual Industry Innovators issue. Summer eBook topics will be formulation, exosomes, biosimilars, and expression systems.
BPI’s publication schedule usually doesn’t allow us to highlight pressing news, which is the purview of our BioProcess Insider newsletter. So when we received two timely papers recently, we were delighted to have a chance to share them with you. Both explore current responses to COVID-19 that will have lasting implications.
Abdi Ghaffari et al. delineate the more than 60 companies currently selling or working on coronavirus diagnostic tests and then highlight what challenges remain for those developers. I want ...
Much has changed since large-scale single-use biomanufacturing equipment was introduced some 15 years ago. Since then, these materials have become accepted and established in production and downstream bioprocessing. Concerns about the environmental impact of single-use (SU) biomanufacturing equipment have become more prevalent as our environmental awareness has increased and related concerns have become more urgent (
1
). For example, many recommendations and even laws have emerged regarding plastic convenience packaging and products (
2
,
3
). People have become more sophisticated in appreciating the distinctions and trade-offs in the pressures upon our air, water, and land. And we appreciate that, beyond passion and resolve, a science-based approach is required to design strategies for a circular economy. We must better understand the implications of industrial activities to reduce environmental stress caused by manufacturing, use, and disposal of single-use biomanufacturing equipment. Tools such as li...
Figure 1: The original Wave Biotechnology rocking bioreactor (left) and Cytiva’s ReadyToProcess WAVE 20 bioreactor (right)
Over the past 10 years, a number of developments in disposable (limited use) and single-use technologies (SUTs) have been made for different bioprocess operations. Until recent years, much of the industry’s process equipment was sterilized using thermal methods such as autoclaving. Most equipment was reusable and required cleaning and sterilization before use. Such processes required validation and expensive and time-consuming resources. Production facilities relied on hard-piped, inflexible equipment such as large stainless-steel bioreactors and holding tanks. However, advanced SUTs now enable fast turnaround between batches and products, with rapid setup, increased flexibility, and fewer resources (compared with stainless-steel systems) required for cleaning, steaming, and cleaning validation. As a result, capital cost is reduced while speed to market authorization is increased.
Fig...
Life-science companies often are cast into the role of the “canary in the coal mine” — the first parties to be targeted and hit by lawsuits. Such companies depend on discovery, trial and error, and ultimately efficacy. None of that is a sure bet. At the same time, life-science companies are raising funds constantly to finance their work. Investors and lenders seeing less-than-projected or even “expected” results might sue directors and officers for mismanagement, misrepresentation, or misleading financial statements.
This is true in cases with directors and officers (D&O) liability claims because a company’s financial prospects can be volatile and because investors often are overoptimistic. A company also can overstate product success and/or prospects. Care is needed in crafting D&O policy language to prevent coverage pitfalls. One example is the “insured versus insured” exclusion by which investors sitting on a board of directors may find their claim outside the scope of coverage.
It’s also not good enou...
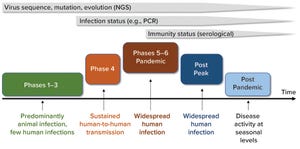
The ongoing coronavirus disease 2019 (COVID-19) outbreak caused by a novel coronavirus (SARS-CoV-2) is posing a great threat to global public health and economies. Accurate and rapid detection of the SARS-CoV-2 virus and diagnosis of infection status will play a critical role in understanding the disease, selecting appropriate treatments, controlling the spread, and developing informed back-to-work policies.
Figure 1: Timeline shows the value of diagnostic tests at different phases of the COVID-19 pandemic. Next-generation sequencing (NGS) played a key role in identifying novel viruses and potential molecular targets for the development of diagnostic tests and antiviral therapeutics. Molecular diagnostic tests (e.g., polymerase chain reaction, PCR) play an important role in detecting an individual’s infectious status and guiding pandemic control strategies. Serological tests can detect individuals with protective immunity against COVID-19 disease (immunity status) and guide back-to-work policies during th...
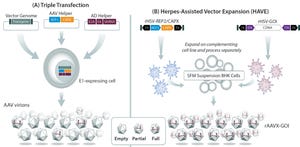
Adenoassociated virus (AAV) often serves as a vector for gene therapies. (www.istockphoto.com)
We might not associate the jazz queen Ella Fitzgerald with 21st-century gene-based therapies, but the First Lady of Song was on to something back in 1939 when she sang “’T’Ain’t What You Do (It’s the Way That You Do It).” Although demonstrating the safety and efficacy of gene-based therapies in rigorous clinical trials is essential for gaining product approval from regulators, doing the bare minimum is insufficient. The way that such products are produced also matters. Manufacturing processes and protocols that assure safety, purity, and consistency of gene-based therapies are critical to the approval process, whereas a cost-effective manufacturing process is essential for commercial success.
US Food and Drug Administration (FDA) approvals of Luxturna (voretigene neparvovec-rzyl) for treatment of RPE65 mutation-associated retinal dystrophy and Zolgensma (onasemnogene abeparvovec-xioi) for SMN1-related spinal mus...
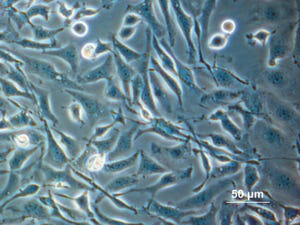
Phase-contrast microscopy image of Chinese hamster ovary (CHO) cells (https://en.wikipedia.org)
Development of a safe and high-quality Chinese hamster ovary (CHO) cell line is of paramount importance for the chemistry, manufacturing, and controls (CMC) portion of studies that support investigational new drug (IND) applications (
1
,
2
). Desirable attributes of a CHO cell line include its ability to
A CHO cell line also should be scalable to high-capacity culture processes and meet required testing standards (e.g., identity, absence of viral or mycoplasma contaminants, and so on).
The first three attributes above strongly depend on molecular characteristics of the expression vector, whose origin and methods of DNA preparation must be reported (
3
). The other two attributes arguably are influenced by laboratory-scale processes and controls incorporated during process design and qualification activities (
4
,
5
).
Furthermore, experimental approaches are likely to change among monoclonal antibodies (MAbs...
Sponsored Content
Astrea Bioseparations is the only adsorbent supplier that can discover new affinity ligands designed to bind selectively to a molecule of interest or specific impurity, develop efficient purification adsorbents and downstream methods, and deliver industrial-scale adsorbents (up to 1,000-L batch sizes) as loose slurry or in good manufacturing practice (GMP)-ready columns. With over 30 years of experience in development of affinity products and design and manufacture of new custom adsorbents, Astrea Bioseparations is a world leader in its field. The company offers an extensive range of off-the-shelf bioseparation products for recovery and purification of biologicals and removal of specific impurities — as well as extensive purification services, including state-of-the-art ligand screening technologies, custom-designed chromatography adsorbents, process development, and large-scale adsorbent manufacturing using the proprietary PuraBead agarose base matrix.
Early stage application of affinity chromatography i...
A key challenge for companies involved in drug development is to meet the highest standards of sterile design and reliability. In this context, magnetic mixers offers many advantages for aseptic stirring processes compared to mechanically sealed agitators.
ZETA not only supplies magnetic agitators for new bioreactors, but also supports its customers through the entire retrofitting process, from feasibility studies at the beginning to full process qualification and validation at the end.
“Combat the risk of batch contamination in bioreactors and mixing vessels by replacing mechanically sealed agitators with magnetically driven technology,” comments Nicole Zangl, Senior Expert and Product Developer for Magnetic Mixers at ZETA.
Nicole Zangl informs on main advantages of magnetic agitators and illustrates the easy retrofit from conventional to the state-of-the-art magnetic mixers for aseptic stirring. The motivated specialist gives further insights into how ZETA can support the retrofitting process of your bi...
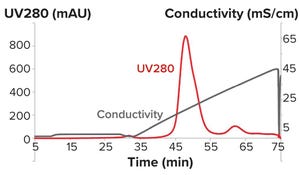
Figure 1: Bind-and-elute chromatogram of a monoclonal antibody purification on SkillPak 5 mL column with Toyopearl NH2-750F resin.
Downstream processing operations make up to 80% of the total costs for processing biotherapeutics. Given the current drive to reduce downstream costs, chromatographers and process engineers will need to streamline processes. Herein, we describe the benefits offered by using Tosoh’s two-step process for purifying monoclonal antibodies (MAbs) and compare that method with the standard industrial process. By combining high-performance protein A capture and a single polishing step on salt-tolerant anion-exchange resin, Tosoh’s approach can reduce downstream costs by 45% and increase production output by 58%.
Material and Methods
Resins and Prepacked Columns:
Toyopearl AF-rProtein A HC-650F is a high-capacity protein A resin for MAb purification. This resin exhibits dynamic binding capacity (DBC) of 70 g/L at five minutes of residence time.
The Toyopearl NH2-750F salt-tolerant anio...
Sue Pearson is an international science writer for the biotechnology, life sciences, and pharmaceutical industries.
In the current coronavirus pandemic, the ability to scale up and produce viral-based vaccines (attenuated viral vaccines, inactivated viral vaccines, and viral vector vaccines) quickly and in large quantities has never before been more relevant. For viral-based vaccines that can be produced by adherent or suspension cell culture, process intensification — in which cell culture, for example, is optimized to produce higher viral titers using the same process equipment — offers a strategy to produce larger numbers of doses in smaller production facilities worldwide, thus reducing overall cost. But what’s stopping researchers from doing this, and what technologies should they be developing to make process intensification a reality?
Science writer Sue Pearson spoke with vaccine experts Sandy Douglas, Piergiuseppe Nestola, Cristina Peixoto, António Roldāo, and Ricardo Silva to find out what intens...
Thomas Gabriel
(director of strategy and business development at Fujifilm Diosynth Biotechnologies, FDB) emphasized patient agency in his 15 April 2020 “Ask the Expert” presentation on innovations in
finished-goods solutions
. Facilitating self-administration significantly benefits patients with chronic conditions, and new delivery technologies are making drug products increasingly easy to handle and with increasingly accurate dosing. Gabriel explored how single-use autoinjectors and prefilled syringes, needle shields, on-body delivery systems, and digital monitors are improving drug-delivery safety and efficacy while enabling patients to take charge of their own health.
Gabriel’s Presentation
Nobody chooses to develop a chronic condition. Thus, finished-goods solutions should enable patients to take their medications and then disengage from their diseases as much as possible to go about living life. Emerging drug-delivery formats are making that happen. Many products still come in vials, which can hind...
Enzyme-linked immunosorbent assays (ELISAs) remain the industry standard for process monitoring and lot-release testing for host-cell proteins (HCPs), but researchers need orthogonal tests to confirm that an ELISA is fit for purpose. On 22 April 2020,
Eric Bishop
(vice president of research and development at Cygnus Technologies) delivered an “Ask the Expert” presentation about his company’s solution: mass spectrometry (MS) preceded by a novel
Antibody Affinity Extraction (AAE) technique
. Bishop explained that an AAE step can magnify a sample’s HCP content and that such enrichment can hone subsequent MS.
Bishop’s Presentation
Currently, ELISAs are the only available method for measuring ng/mL HCP levels in mg/mL levels of target protein. Unfortunately, such testing does not reveal what HCPs the assay reacted to in sampled drug substance. Thus, orthogonal evaluation is necessary. Historically, researchers relied on two-dimensional (2D) Western-blot analysis for support, but that approach inconsistently...
The novel coronavirus disease 2019 (COVID-19) pandemic has every industry seeking out ways to accomplish time-sensitive activities using a number of virtual approaches. This is certainly true in the biopharmaceutical sector, in which good manufacturing practice (GMP) audits are required to manufacture medicinal drug products for human use. Examples include supplier/vendor audits, mock inspections, and preapproval and prelicense inspections (PAIs and PLIs) conducted by sponsors and regulatory authorities. Auditors usually perform such activities on site and only sometimes remotely. In the latter case, the process typically involves reviewing documents that are shared electronically. With the current embargo on travel and the need for “social distancing,” on-site visits are impossible. Companies and auditors alike now need to adopt new methods for conducting “on-site” audits without being physically present. We refer to this concept as a
virtual audit
. Here, we propose a process and provide guidance for a...
Sponsored Content
Marie-Laure Collignon and Shahin Heshmatifar
Technology transfer is a key milestone in the journey from discovery to full-scale good manufacturing practice (GMP)-compliant manufacturing. Navigating this step while preventing unforeseen issues that can create costly delays is supported best by combining knowledge of a given process with understanding of the technological capabilities.
Different applications have different needs. Some challenges and goals are common to bioreactor processes for suspension and adherent cell culture for production of viral vectors, monoclonal antibodies (MAbs), other recombinant proteins, and vaccines. All those applications need improvements in productivity, yields, and efficiency while maintaining critical quality attributes (CQAs) established at small scale. Such goals can be met only through optimizing the right technology and using it in a way that delivers required performance. Here we discuss how specialist teams at Pall Biotech approach technology transfer and work wit...