Trends in COVID-19 Diagnostic Test DevelopmentTrends in COVID-19 Diagnostic Test Development
The ongoing coronavirus disease 2019 (COVID-19) outbreak caused by a novel coronavirus (SARS-CoV-2) is posing a great threat to global public health and economies. Accurate and rapid detection of the SARS-CoV-2 virus and diagnosis of infection status will play a critical role in understanding the disease, selecting appropriate treatments, controlling the spread, and developing informed back-to-work policies.
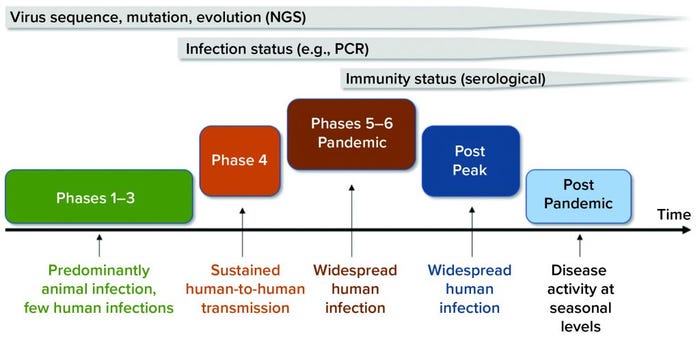
Figure 1: Timeline shows the value of diagnostic tests at different phases of the COVID-19 pandemic. Next-generation sequencing (NGS) played a key role in identifying novel viruses and potential molecular targets for the development of diagnostic tests and antiviral therapeutics. Molecular diagnostic tests (e.g., polymerase chain reaction, PCR) play an important role in detecting an individual’s infectious status and guiding pandemic control strategies. Serological tests can detect individuals with protective immunity against COVID-19 disease (immunity status) and guide back-to-work policies during the postpeak phase. (viral pandemic phases from www.who.int/csr/disease/swineflu/phase)
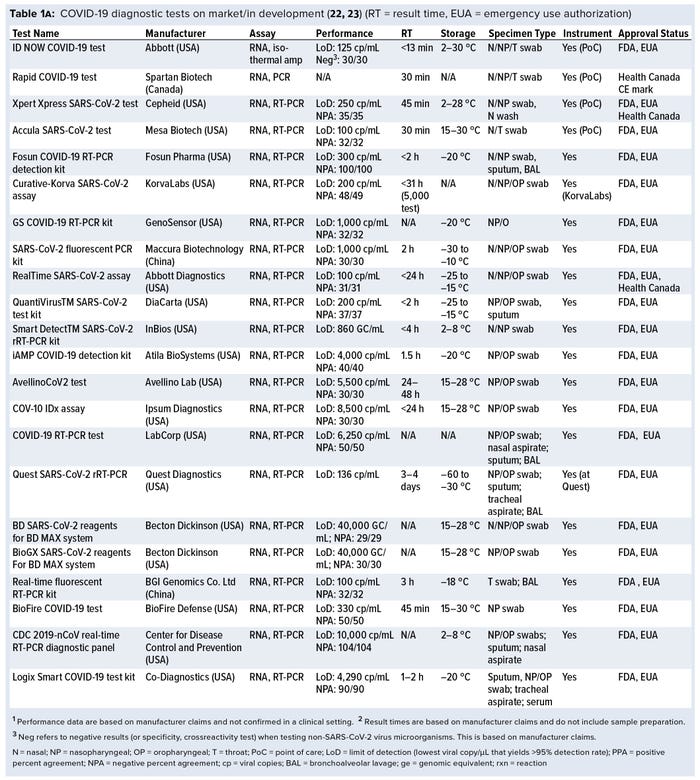
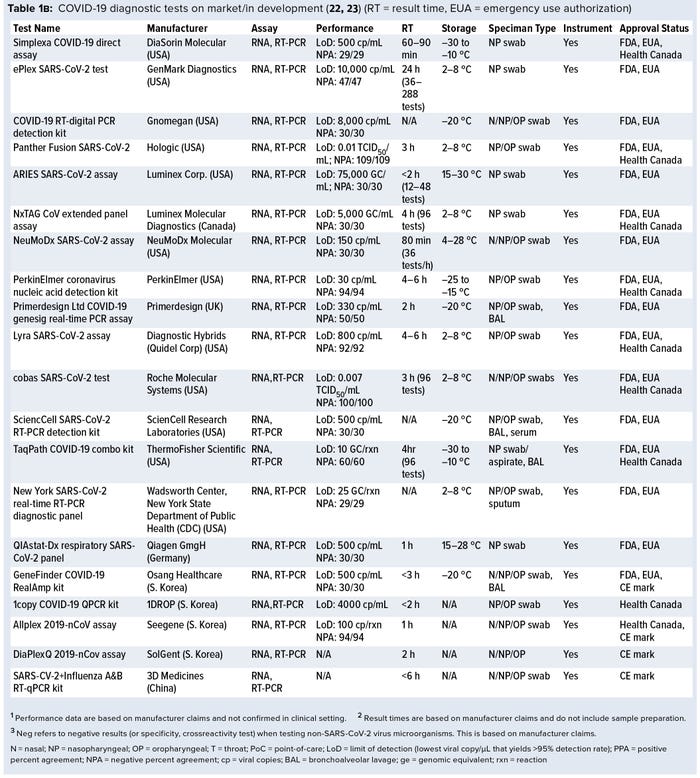
Currently, a review of ongoing efforts in the development of COVID-19 diagnostic tests is lacking. For this review, we collected data on more than 336 COVID-19 diagnostic tests from several medical-device regulatory agencies, including the US Food and Drug Administration (FDA), European Union, and Health Canada. Next, we performed an in-depth assessment of 50 nucleic-acid–based (e.g., polymerase chain reaction, PCR), 14 serological, and one antigen-based COVID-19 diagnostic tests currently approved by the FDA and Health Canada and with reported performance data. Key features such as limit of detection (LoD), crossreactivity, time-to-result, storage conditions, sample type, instrument requirement, and regulatory approval status were reviewed. Our findings identify a significant gap in the development of rapid, accurate, and simple point-of-care (PoC) COVID-19 diagnostic tests and a lack of standardized validation protocols with access to appropriate SARS-CoV-2 clinical samples.
Background
In December 2019, a cluster of cases with severe respiratory illness causing pneumonia and death were reported in Wuhan, China. As the disease spread swiftly, the causative agent was confirmed to be a novel coronavirus. It was named SARS-CoV-2 because of its phylogenetic similarity to severe acute respiratory syndrome (SARS) coronavirus from a 2002 viral outbreak (1). The genomic sequence of isolated SARS-CoV-2 soon was released to public databases (1), allowing for development of molecular-based COVID-19 diagnostic tests.
As we entered the peak phase of an unprecedented global pandemic (Figure 1) and in the absence of an effective therapy or vaccine for COVID-19, aggressive diagnostic testing strategies became essential. They allow researchers to understand how the pandemic is progressing, how to deploy effective countermeasures such as contact tracing and isolation, how to control and minimize the second wave of the pandemic, how to assess protective immunity status in populations, and how to initiate informative back-to-work policies and restart the economy (2). To address those challenges, a clear understanding of available COVID-19 diagnostic tests and technological advances under development is paramount.
Current viral diagnostic tests can be divided into two broad categories: those that detect the virus and those that detect the host’s response to the viral infection (2). Each category can play a critical and complementary role in response to COVID-19 diseases at different stages of the pandemic (Figure 1). Below, we overview the technological platforms available for development of SARS-CoV-2 viral diagnostic tests.
COVID-19 Diagnostic Test Platforms
Current molecular-based assays that can detect the presence of SARS-CoV-2 virus directly in patient samples include genomic sequencing, nucleic acid application, and antigen-based assays (3). Although culture techniques can expand the virus for detection purposes, safety concerns restrict the practice of such methods for novel viral infections. The other broad test category is serological assays to detect host immune response to viral infection (3). The following is a brief description of each test and comparison of characteristics of each diagnostic technology platform (Figure 2).
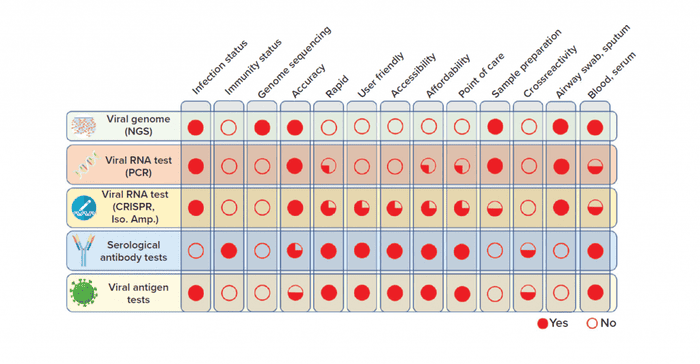
Figure 2: Overview of COVID-19 diagnostic test technologies (NGS = next-generation sequencing; Iso. Amp. = isothermal amplification)
Viral Genome Sequencing: Deep-sequencing methods such as next-generation sequencing (NGS) have revolutionized virology and the detection of novel emerging viruses (1). However, high costs associated with instruments, reagents, and specially trained staff in addition to the complexity of data analysis have prevented application of NGS technology as a rapid diagnostic test. Nonetheless, the periodic use of NGS to sequence novel viruses such as SARS-CoV-2 in clinical samples will be critical in our understanding of virus evolution and identification of novel targets for development of diagnostic tests and antiviral therapeutics.
Nucleic-Acid Amplification Test: Currently, the gold-standard test for direct detection of SARS-CoV-2 is based on PCR technology. PCR is used to amplify minute amounts of viral genetic material in clinical samples to achieve the needed high rate of sensitivity and specificity in conducting diagnostic tests. However, current PCR tests require time-consuming sample preparation, sophisticated instruments, and highly trained staff. So several companies have developed smaller and more user-friendly PCR systems for rapid detection and PoC diagnosis (e.g., Cepheid, GenMark, Mesa Biotech, Table 1a–c). Alternatively, isothermal amplification methods that do not require PCR instruments — including loop-mediated isothermal amplification (LAMP) (e.g., Abbott’s ID NOW COVID-19 test, Table 1a), rapid diagnostic tests based on clustered regularly interspaced short palindromic repeats (CRISPR) (e.g., Mammoth Biosciences, and Sherlock Biosciences, Table 1c) and RNA/DNA methods — are examples of innovative technologies under development in this category (4, 5).
Viral antigen tests exploit the high binding affinity between an antibody and a unique viral antigen (typically protein targets) to detect the presence of a specific virus in fluid samples. Coronaviruses consist of four structural proteins: spike (S), membrane (M), envelope (E), and nucleocapsid (N) proteins. Cell-surface markers such as the S protein and other structural proteins are primary targets in the SARS-CoV-2 virus for development of antigen-based tests. Lateral-flow assays (LFAs) commonly are used to develop low-cost, simple, rapid, and portable antigen-based diagnostic tests (6).
In LFA technology, a monoclonal antibody (MAb) against a specific viral antigen is secured on an absorbing surface that captures the virus molecule in a fluid sample. The molecule then interacts with a second detector antibody (linked to a colorimetric, fluorescent, or colloidal gold probe) to register a positive result (6). However, because the approach has lower sensitivity and specificity than PCR (7), antigen-based tests have not been the main focus of COVID-19 diagnostic test development. Sona Nanotech is collaborating with Cytiva to develop a PoC antigen-based test that detects the S1 domain of SARS-CoV-2 spike protein in respiratory samples (Table 1a–c).
Serological antibody tests monitor the host immune response to COVID-19 infection by detecting SARS-CoV-2 specific antibodies IgG and IgM in the host blood (or serum or plasma). It is important to note that it takes 7–14 days after COVID-19 infection for antibody seroconversion, and for the SARS-CoV-2-specific IgG/IgM antibodies to become detectable in host blood (8). Thus, serological tests are unsuitable for early diagnosis, but they can play a critical role in later phases of the COVID-19 infection (Figure 1) to identify individuals with protective immunity against COVID-19. Typically, serological tests also use an LFA-based technology to detect IgG/IgM in a few drops of blood and are low-cost, simple, rapid, and portable diagnostic tests.
Developing reliable serological tests requires overcoming a number of challenges. One limitation is the crossreactivity of host antibodies with non–SARS-CoV-2 viruses because of a high number of sequence and structure similarities among the coronaviruses (9). To address that, some manufacturers (e.g., PharmACT, Table 1) are targeting multiple regions of SARS-CoV-2 antigens to minimize false-positive results produced by crossreaction (10). Another limitation is correlation with severity of COVID-19 disease. That means that patients with mild or asymptomatic diseases might have a negative test result.
Finally, not all antibodies developed by a host will neutralize the SARS-CoV-2 virus, and we currently do not know how long neuralization antibodies will persist to be effective against reinfection (10). Current serological tests will facilitate diagnosis, but their utility to test for immunity remains to be proven.
To assess the value of each diagnostic test technology discussed above, we compared key characteristics and features side by side (Figure 2).
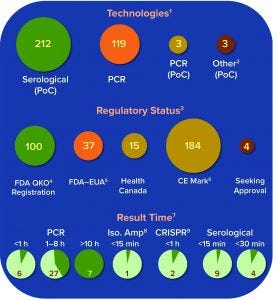
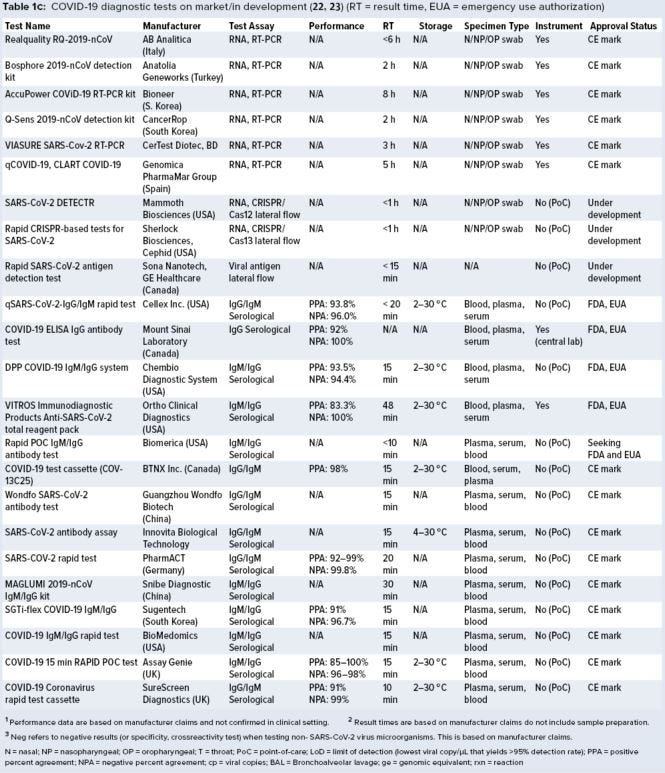
Figure 3: Summary of current COVID-19 diagnostic tests on the market or in development; number of tests under each technology platform, regulatory status, and result time is shown.
Overview of Current COVID-19 Diagnostic Tests
A growing number of companies are developing innovative COVID-19 diagnostic technologies to address the need for highly accurate, rapid, and accessible tests so that countries can implement broad testing strategies. We collected data on 336 COVID-19 diagnostic tests from the FDA Serological Medical Device Registration database (11), the FDA COVID-19 diagnostic tests emergency use authorization (EUA) database (12), Health Canada COVID-19 Diagnostic Device Application database (13), and European Commission COVID-19 test devices database (14). We also assessed in-depth key features of 65 COVID-19 tests with an accessible product-information package and performance data. Table 1a–c summarizes key characteristics, including manufacturer, sensitivity or LoD data, specificity or crossreactivity data (negative test), time to result, storage conditions, sample type, instrument requirement, PoC capability, and regulatory status. Standardized protocols for performance validation and access to SARS-CoV-2 clinical isolates currently are limited, and results in Table 1 are based solely on manufacturers’ claims. Figure 3 overviews the data presented in the table, including the number of tests under each category, their regulatory status, and time-to-result data.
Regulatory Approval of COVID-19 Diagnostic Tests
In the United States on 16 March 2020, the FDA issued an immediately in-effect guidance for clinical laboratories, commercial manufacturers, and FDA staff titled Coronavirus Disease-2019 Tests During the Public Health Emergency (15). The guidance covers validation recommendations, instructions for the FDA notification, and EUA requests for molecular, antigen-detection, and serological diagnostics tests. Availability of testing has been a choke point in the US response to the COVID-19 pandemic. In response, the FDA has relaxed requirements allowing test manufacturers to distribute their molecular tests commercially after validation is complete and while they are preparing their EUA requests (16). Further, the FDA has allowed serological tests with qualitative detection claims to enter the market without an EUA.
Initially, it appeared that the benefits of access to testing outweighed the risks of potential performance issues. However, on 4 May 2020, the FDA revised its policy on antibody-based tests. The agency now requires all manufacturers to submit an EUA request with validation data within 10 business days of the date they notified the FDA or from the date of the new policy (16). The FDA also revised the Policy for Coronavirus Disease-2019 guidance document to include the new requirements and an EUA template specific to serological tests (16).
In Canada on 18 March 2020, the Minister of Health approved an interim order (IO) to expedite the review of medical devices used to diagnose, treat, or prevent COVID-19 (17). An IO is one of the fastest regulatory mechanisms available to address large-scale public health emergencies. Under an IO, a manufacturer is required to submit an abbreviated application to support the safety, effectiveness, and quality of its medical devices. Health Canada has published two guidance documents to assist manufacturers in the application process. The first guide covers applications for medical devices under the IO for use in relation to COVID-19 (18). The second is on the requirements for serological antibody tests submitted under the COVID-19 IO (19).
Health Canada is waiving fees associated with IO applications, and manufacturers applying through this pathway will not have to hold medical-device single-audit program (MDSAP) certification. This strategy is the most effective pathway for manufacturers to apply for their COVID-19 device authorization in Canada.
On 15 April 2020, the European Commission published guidelines on COVID-19 in-vitro diagnostic tests and their performance. The objectives were to outline the regulatory context of COVID-19-related diagnostic testing devices in the European Union and to provide an overview of different types of tests and their purposes (20). The guideline also includes considerations on device performance and validating that performance. In brief, Directive 98/79/EC on in-vitro diagnostic medical devices (IVD) currently applies to COVID-19 tests (21). Device manufacturers must comply with relevant provisions of this directive to market their products. Specifically, they must provide evidence that tests are safe and that they perform as intended, by demonstrating compliance with requirements in Annex I of Directive 98/79/EC. When a device manufacturer considers that the test complies, it can be CE marked, according to the directive. Self-tests must first have notified body conformity assessment. At present, no self-tests have been CE marked and a number of EU member states are not permitting their use. However, the directive provides a means whereby marketing of a product deemed important to a public health crisis can be made before all CE marking commitments are obtained. To benefit from such derogation, a manufacturer must approach the competent authority in the member state where it has its EU legal base.
Robust, Early Testing Is Key
As countries struggle to contain the COVID-19 pandemic, the importance of robust testing strategies is becoming apparent. In countries such as South Korea, Singapore, and Germany, aggressive and early COVID-19 testing seems to have contained the spread of the infection and has drastically lowered death rates compared with countries that responded less promptly. A robust diagnostic testing policy also can lead to improved containment of a second wave in the postpeak phase of COVID-19 pandemic.
Large-scale serological antibody testing to assess protective immunity status in communities has to be a key part of every back-to-work policy. Because novel pandemic viruses can become highly contagious, a significant gap in the development of rapid, accurate, and simple PoC diagnostic tests must be addressed to implement meaningful regional or national testing policies and reduce the workload at centralized laboratories.
The number of tests approved under emergency-use programs is mounting. New challenges in the short term include increased understanding of performance variability with existing diagnostics, standardization of performance test protocols, access to clinical isolates of virus, shortened time to results, and robust supply lines to increase accessibility. A coordinated partnership among government, industry, and academia is needed to address these challenges promptly. Finally, we must remind ourselves how fortunate we are to live in a time with access to incredible and innovative technologies that can tackle these challenges.
References
1 Zhu N, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 382(8) 2020: 727–733; https://doi.org/10.1056/NEJMoa2001017.
2 Patel R, et al. Report from the American Society for Microbiology COVID-19 International Summit, 23 March 2020: Value of Diagnostic Testing for SARS-CoV-2/COVID-19. mBio 11(2) 2020; https://doi.org/10.1128/mBio.00722-20.
3 Ahn DG, et al. Current Status of Epidemiology, Diagnosis, Therapeutics, and Vaccines for Novel Coronavirus Disease 2019 (COVID-19). J. Microbiol. Biotechnol. 30(3) 2020: 313–324; https://doi.org/10.4014/jmb.2003.03011.
4 Broughton JP, et al. Rapid Detection of 2019 Novel Coronavirus SARS-CoV-2 Using a CRISPR-Based DETECTR Lateral Flow Assay. medRxiv 27 March 2020; https://doi.org/10.1101/ 2020.03.06.20032334.
5 Udugama B, et al. Diagnosing COVID-19: The Disease and Tools for Detection. ACS Nano 14(4) 2020: 3822–3835; https://doi.org/10.1021/acsnano.0c02624.
6 Koczula KM, Gallotta A. Lateral Flow Assays. Essays Biochem. 60(1) 2016: 111–120; https://doi.org/10.1042/EBC20150012.
7 Ghebremedhin B, et al. Comparison of the Performance of the Rapid Antigen Detection Actim Influenza A&B Test and RT-PCR in Different Respiratory Specimens. J. Med. Microbiol. 58(Pt 3) 2009: 365–370; https://doi.org/10.1099/jmm.0.004358-0.
8 Wolfel R, et al. Virological Assessment of Hospitalized Patients with COVID-2019. Nature 1 April 2020; https://doi.org/10.1038/s41586-020-2196-x.
9 Meyer B, et al. Serological Assays for Emerging Coronaviruses: Challenges and Pitfalls. Virus Res. 194, 2014: 175–183; https://doi.org/10.1016/j.virusres.2014.03.018.
10 Yan Y, et al. Laboratory Testing of SARS-CoV, MERS-CoV, and SARS-CoV-2 (2019-nCoV): Current Status, Challenges, and Countermeasures. Rev. Med. Virol. 2020: e2106; https://doi.org/10.1002/rmv.2106.
11 FDA Serological Tests Registration Database. US Food and Drug Administration: Rockville, MD, 2020; https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRL/rl.cfm?start_search=91&establishmentName=®Num=&StateName=&CountryName=&RegistrationNumber=&OwnerOperatorNumber=&OwnerOperatorName=&ProductCode=QKO&DeviceName=&ProprietaryName=&establishmentType=&PAGENUM=10&SortColumn=EstablishmentName20%25ASC.
12 FDA COVID-19 Diagnostic Tests Emergency Use Authorization Database. US Food and Drug Administration: Rockville, MD, 2020; https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations#covid19ivd.
13 Health Canada COVID-19 Diagnostic Device Application Database. Health Canada: Ottawa, Canada, 2020; https://www.canada.ca/en/health-canada/services/drugs-health-products/medical-devices/covid-19/diagnostic-devices-applications.html.
14 European Commission COVID-19 Tests Database. European Commission: Brussels, Belgium, April 2020; https://ec.europa.eu/docsroom/documents/40805.
15 Immediately in Effect Guidance for Clinical Laboratories, Commercial Manufacturers, and Food and Drug Administration Staff: Policy for Coronavirus Disease-2019 Tests During the Public Health Emergency. Food Drug Administration Center for Devices and Radiological Health: Rockville, MD, 2020; https://www.fda.gov/regulatory-information/search-fda-guidance-documents/policy-coronavirus-disease-2019-tests-during-public-health-emergency-revised.
16 Policy for Diagnostic Tests for Coronavirus Disease-2019 during the Public Health Emergency. Food Drug Administration: Rockville, MD, March 2020; https://www.fda.gov/regulatory-information/search-fda-guidance-documents/policy-diagnostic-tests-coronavirus-disease-2019-during-public-health-emergency.
17 Interim Order Respecting the Importation and Sale of Medical Devices for Use in Relation to COVID-19. Health Canada: Ottawa, Canada, March 2020; https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/announcements/interim-order-importation-sale-medical-devices-covid-19.html.
18 Applications for Medical Devices Under the Interim Order for Use in Relation to COVID-19: Guidance. Health Canada: Ottawa, Canada; https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/announcements/interim-order-importation-sale-medical-devices-covid-19/guidance-medical-device-applications.html.
19 Requirements for Serological Antibody Tests Submitted under the COVID-19 Interim Order: Guidance. Health Canada: Ottawa, Canada; https://www.canada.ca/en/health-canada/services/drugs-health-products/medical-devices/application-information/guidance-documents/covid19-requirements-serological-antibody-tests.html.
20 Guidelines on COVID-19 In Vitro Diagnostic Tests and Their Performance. European Commission: Brussels, Belgium, 2020; https://ec.europa.eu/info/sites/info/files/testing_kits_communication.pdf.
21 Offical Journal of the European Communities. 41(L331). European Commission: Brussels, Belgium, 7 December 1998; https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:1998:331:FULL&from=EN.
22 FDA Emergency Use Authorizations. US Food and Drug Administration: Rockville, MD, 2020; https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations#covid19ivd.
23 Sheridan C. Fast, Portable Tests Come Online to Curb Coronavirus Pandemic. Nature Biotechnol. News 7 April 2020; https://doi.org/10.1038/d41587-020-00010-2.
Corresponding author Abdi Ghaffari is an advisor in scientific affairs at Novateur Ventures, Vancouver, BC, Canada, and an adjunct associate professor at the department of pathology and molecular medicine at Queen’s University, Kingston, ON, Canada. Ian McGill is lead of US diagnostics regulatory affairs, and Ali Ardakani is founder and managing director, both at Novateur Ventures.
You May Also Like






