Every now and then in the biopharmaceutical industry, a technology or concept seems to take hold and suddenly appears everywhere — in print, at conferences, in workshops and online media offerings, and as part of new product launches. This happened in the 1990s when process validation was (seemingly suddenly) the theme of the day, and it happened briefly later that decade when transgenics appeared to be a potential game changer.
Single-use technologies of course precipitated arguably the most widespread set of “disruptions” to accepted ways of approaching development and biomanufacturing processes — with worldwide ramifications. About the same time that disposables began to deserve greater coverage, we also began to see more practical details from biomanufacturers implementing the quality by design (QbD) regulatory initiative. And unlike the situation was 10 years ago, the concept of process analytical technologies (PAT) now actually means something to most people working in the modern biotechnology indus...
WWW.GRAPHICSTOCK.COM
Niche Disease: Autoimmune/Noninfectious Uveitis
Uveitis is the inflammation of the uvea, the pigmented layer that lies between the eye’s inner retina and its outer fibrous layer composed of the sclera and cornea. The most frequent etiologies are autoimmune diseases such as Behçet disease. Noninfectious intermediate and posterior uveitis (NIU) represents 10–15% of uveitis cases, making it the fourth leading cause of blindness in the developed world. About one in 4,500 patients suffer with this condition. Uveitis typically has been treated with glucocorticoid steroids. But every year, an estimated 30,000 US and EU patients with autoimmune uveitis are refractory to approved treatments. If the condition goes untreated, serious complications including blindness can result.
Organizations:
One support group is the Ocular Immunology and Uveitis Foundation (www.uveitis.org). Its mission is to find cures for ocular inflammatory diseases, to correct the worldwide deficit of properly trained ocu...
2,000-L stainless steel bioreactor
As an increasing number of cell therapies move into late-phase trials, developers are considering innovative solutions to address scale-up and commercialization challenges. Many of their questions focus on the technologies and engineering strategies that will be needed to optimize their processes, especially bioreactors.
At the January 2016 Phacilitate Cell and Gene Therapy World conference, Siddharth Gupta, a scientist at Lonza (Walkersville, MD), talked about the effects of upstream process decisions on product quality in his presentation “Bioreactor Manufacturing Platforms: So You Think You Know What It Takes, But Do You Really?”
Difficulties and Risks
What are the essential components of developing cell therapies?
The 19th century was all about chemistry, and the 20th century was all about physics. Many industry experts are anticipating that this century will be all about biology and its intersection with technology. The only way to make that work is to have great b...
WWW.GRAPHICSTOCK.COM
Small-molecule impurities that bind to and copurify with protein biopharmaceuticals traditionally have been removed using bind-and-elute (BE) chromatography. However, that approach may be undesirable for a number of reasons. For instance, it may present a facility-fit challenge or provide a lower process yield than what is acceptable.
A common scenario in which BE chromatography may be undesirable is in removal of unreacted conjugation reagents. Bioconjugates represent an important and growing class of pharmaceuticals that include PEGylated proteins, vaccines, and antibody–drug conjugates (ADCs) (
1
–
8
). The latter, for example, are produced by combining a monoclonal antibody (MAb) or MAb-like molecule with a reactive payload reagent to form a conjugate (
9
). Following that reaction, unreacted payload molecules (“free drug”) must be removed from the process stream to very low levels because they are highly toxic. That is typically achieved using diafiltration, which exploits the si...
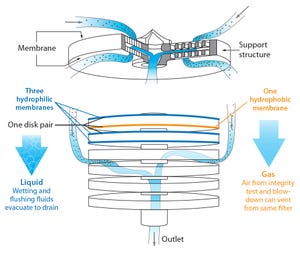
Figure 1: Design and layout of Millidisk and Millipak barrier filters
A number of regulatory guidelines recommend preuse integrity testing of critical sterilizing liquid filters for aseptic processing (
1
–
3
). Before sterilization, a preuse test will confirm that a filter is installed properly and was not damaged during shipment or handling. Performing a preuse test after sterilization detects damage that may have occurred during the sterilization cycle. Testing after sterilization limits risk, so it is a practice applied based on risk assessment. Because it is perceived to reduce business loss risk, preuse post-sterilization integrity testing (PUPSIT) is a current industry practice especially in manufacturing products that will be marketed in the European Union (EU).
Unfortunately, it can be difficult to perform a PUPSIT without breaching system sterility. A number of methods have been developed for running PUPSIT and performing line conditioning without compromising sterility. Such methods use a flush...
Process intensification through continuous manufacturing has been practiced in the chemical, petrochemical, and food industries for years and has gained much interest among biopharmaceutical manufacturers (
1
). Key drivers encouraging biomanufacturers of therapeutic molecules to convert batch processes into continuous operation include flexibility, productivity, cost effectiveness, and product consistency.
Continuous upstream processing has been demonstrated for the manufacture of a broad range of molecules, including complex/labile proteins such as enzymes (
2
) and monoclonal antibodies (
3
). Recent publications have reported successful application of single-use technologies for perfusion processes as a next logical development step in combining flexibility with process efficiency (
4
).
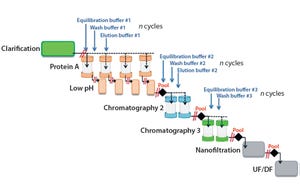
For continuous downstream processing, however, additional efforts will be required to attain a similar level of maturity and finally provide for robust continuous unit operations. Current concepts for process intensif...
Photo 1: Standard one-bag position in chamber
It is well understood that freeze– thaw processes affect the product quality of biopharmaceuticals (
1
–
3
). It has been reported that there is no consistent method of controlled freezing and thawing rates for biological formulations (
4
). Traditionally, ultralow temperature storage chambers that were not designed for freezing have been used to provide an energy state for the environment surrounding the product with very little excess capacity to change the state of the product.
This study details a consistent method for controlled-rate freezing and thawing. It also includes the effect of load and container position on freeze rates. The freeze–thaw controlled-rate chamber used in this study was a model 4002 manufactured by Farrar Scientific. It permits rapid, uniform bulk freezing and/or thawing of products with temperature ranges from +40 °C to –80 °C. It has a minimum of 1.7 kW of net cooling and heating capacity over the entire temperature range and offer...
A YouTube video shows the RAMbio mixing system in action. (WWW.YOUTUBE.COM/WATCH?V=AFUE56BV85G)
Production of recombinant proteins usually happens in suspension cultures, with oxygen limitation playing a major role. Oxygen and nutrition feeds are of great significance to aerobic suspension cultures. Oxygen is often the controlling factor in orbital shaken systems because oxygen transfer occurs only through diffusion, which is limited by gas-exchange surface and mixing characteristics.
Here, we compare growth characteristics of microbial cultures in a standard shaken incubator with those of cultures in a RAMbio fermentation system, paying particular attention to oxygen transfer. The most common approach for improving oxygen transfer is to increase the exchange surface. In typical orbital shaken incubators, mixing is achieved through horizontal two-dimensional movement. Laminar flow is reduced by changing orbital frequencies or with integrated baffles in the lower part of each flask allowing eddy formation....
The next 12–18 months could be a critical time for biosimilars in the United States (
1
). This product class has grown rapidly since passage of the Biosimilars Price Competition and Innovation Act (BPCIA) of 2010. That landmark legislation allowed for biosimilar market approvals based on previously approved “reference products,” creating an expedited pathway that reduces biosimilar development costs and speeds regulatory review so patients get faster access to essential medicines. Increased competition from biosimilars could save the US healthcare system billions of dollars. Both regulators and the judiciary are now revisiting important aspects of BPCIA, and their decisions will help determine the full market potential of this product class.
Regulatory Questions
In March 2015, the US Food and Drug Administration (FDA) approved its first biosimilar product, more applications have been filed, and companies are investing heavily in development. Against this backdrop, the FDA is considering several key quest...