Orbital Shaking and Acoustic-Resonance Mixing: Comparing Culture CharacteristicsOrbital Shaking and Acoustic-Resonance Mixing: Comparing Culture Characteristics
May 11, 2016

A YouTube video shows the RAMbio mixing system in action. (WWW.YOUTUBE.COM/WATCH?V=AFUE56BV85G)
Production of recombinant proteins usually happens in suspension cultures, with oxygen limitation playing a major role. Oxygen and nutrition feeds are of great significance to aerobic suspension cultures. Oxygen is often the controlling factor in orbital shaken systems because oxygen transfer occurs only through diffusion, which is limited by gas-exchange surface and mixing characteristics.
Here, we compare growth characteristics of microbial cultures in a standard shaken incubator with those of cultures in a RAMbio fermentation system, paying particular attention to oxygen transfer. The most common approach for improving oxygen transfer is to increase the exchange surface. In typical orbital shaken incubators, mixing is achieved through horizontal two-dimensional movement. Laminar flow is reduced by changing orbital frequencies or with integrated baffles in the lower part of each flask allowing eddy formation. Such strategies effectively increase the exchange surface and therefore the oxygen transfer rate.
The RAMbio system introduced by Applikon Biotechnology forms eddies through one-dimensional vertical movement induced by an acoustic resonance system. Located under the mixing platform, it operates at a frequency of 58–66 Hz. The proprietary Resonant Acoustic mixing technology at the heart of this system is a distinct approach to mixing and dispersion from conventional impeller agitation or ultrasonic mixing. This uses low-frequency, high-intensity acoustic energy to create a uniform shear field throughout an entire mixing vessel. An oscillating platform transfers energy to flasks and thereby effects mixing within a culture suspension. Simultaneously, oscillations break up gas bubbles to further increase the gas-exchange surface and oxygen-transfer rate. The result is rapid dispersion of material.
Orbital shaking platforms can mix cultures, but they provide no active turnover of a flask’s headspace. As a result, oxygen-depleted headspace gas limits the oxygen uptake rate of growing cultures. A RAMbio system overcomes that limitation by actively replenishing the headspace gas in flask cultures. Its patented OxyPump stopper provides headspace refreshment rates of ≤2 vvm (equivalent to that of stirred-tank bioreactors), providing oxygen transfer rates up to six times greater than orbital shaker platforms can achieve.
Early Validation Study
Applikon Biotechnology and collaborators in Peter Czermak’s laboratory at the Mittelhessen University of Applied Sciences (Gießen, Germany) have undertaken an early comparison of growth characteristics in both a standard shaken incubator and a RAMbio system. We investigated two microorganisms — Escherichia coli BL21 and Pichia pastoris pink — in standard, unoptimized culture media: lysogeny broth (LB) medium for the former and yeast-extract peptone dextrose (YPD) medium for the latter.
Culture media recipes included the addition of yeast extract (5 g/L for LB, 10 g/L for YPD), peptones (10 g/L for LB, 20 g/L for YPD), glucose (5 g/L for LB, 20 g/L for YPD), sodium chloride (5 g/L for LB only) and J-647 antifoaming agent from Struktol GmbH under sterile conditions to distilled water. After preparing inoculation cultures, we initiated fermentation by adding starter cultures to each medium and incubating them overnight in a Multitron Standard incubator from Infors HT at 300 rpm, 30 °C for P. pastoris and 37 °C for E. coli. Parallel cultures ran in a RAMbio system.
Starting cultures were adjusted to an optical density (OD) of 0.1 with a Spectronic 200 photometer from Thermo Scientific set at 600 nm for related measurements. Orbital-shaker cultivation was in 1-L baffled flasks, each containing 200 mL medium. RAMbio cultivation was in 500-mL Erlenmeyer flasks also using 200 mL of medium. These cultures ran in parallel for 25 hours at temperatures specific to each microbe.
Growth Kinetics: Growth-rate determinations are essential for comparing the influence of culture conditions on microbial cultures. So the team measured OD and glucose concentrations twice every hour using 3-mL samples. Readings higher than 0.9 necessitated dilution with medium. Glucose analysis was performed using a Biosen C-Line analyzer from EKF Diagnostics, according to the manufacturer’s online standard operating procedure (SOP) (1). For the exponential growth phase only, the team calculated maximum growth rates using the following equation:
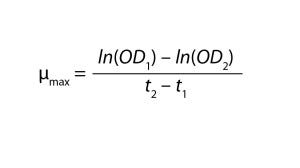

Table 1: OD and maximum growth rate (µmax) during the exponential growth phase of a P. pastoris pink culture
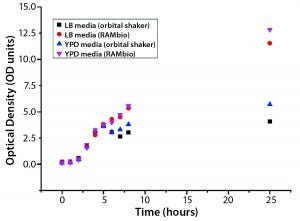
Figure 1: Arithmetic average of optical density (OD) as a function of culture time for Pichia pastoris pink in different shake-flask cultures
Results
Pichia pastoris Pink: Figure 1 illustrates OD as a function of culture time for the P. pastoris pink culture. The team used the same starting density for all four culture conditions. As the figure shows, in all cultures exponential growth followed a brief lag phase. Table 1 lists the OD values at culture’s end (25 h) and maximum growth rates reached during exponential growth. Figure 2 illustrates glucose concentrations for the P. pastoris cultures. Comparing
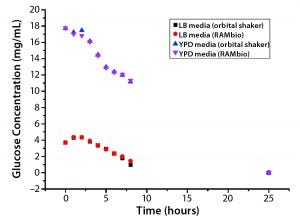
Figure 2: Arithmetic average of glucose concentration over time for P. pastoris pink in different shake-flask cultures
Figures 1 and 2 shows that a decrease in glucose concentration correlates with OD increases over time.
Escherichia coli BL21: Figures 3 and 4 graph OD and glucose concentrations as a function of time for the E. coli cultures. Their growth was analogous to that of P. pastoris pink. Note that comparable glucose consumption in the two culture systems tested leads to very different ODs. RAMbio fermentation produces significantly more biomass because microbes in oxygen-constrained cultures cannot generate as much biomass per unit of glucose concentration. Table 2 shows OD values at the end of these cultures (25 h) and maximum growth rates reached during exponential growth.

Table 2: Optical density and µmax of E. coli BL21 cultures
Independent Confirmation
A recent study provided independent confirmation of the capabilities of resonant acoustic mixing (2). Investigators at the Universidad Nacional Autónoma de México (Mexico City) compared empirical kLa values in a RAMbio E. coli fermentation with those from a standard orbital mixer. The volumetric masstransfer coefficient kLa describes the efficiency of oxygen delivery in a bioreactor for a given set of conditions. That makes it a critical parameter in microorganism growth and viability.
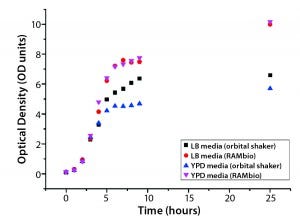
Figure 3: Arithmetic average of OD as a function of culture time for Escherichia coli BL21 in different shake-flask cultures
Because kLa depends on several conditions, the researchers compared different shaking frequencies, nominal flask volumes, temperatures, and filling volumes. They found strong correlation between kLa and culture conditions in both systems. Specifically, kLa was strongly dependent on agitation. Throughout normal agitation levels in both systems, however, RAMbio kLa values were significantly higher than those achieved with orbital mixing. Overall, the maximum kLa observed with a New Brunswick Scientific C251 orbital shaker was 131.3 ± 5.1 h−1. The maximum kLa for the RAMbio system was more than three times greater, 435.4 ± 11.7 h−1.
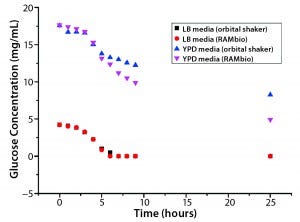
Figure 4: Arithmetic average of glucose concentration over time for E. coli BL21 in different shake-flask cultures
To further investigate differences between the two fermentation systems, these investigators compared E. coli growth kinetics, glucose uptake, dissolved oxygen tension (DOT), and organic acids production in the two systems at equivalent initial kLa values. They found that growth kinetics were comparable at an initial kLa of 46 h−1, but not at 92 h−1. At that point, differences in DOT and culture parameters were observed mainly in growth rate and biomass yield from glucose. The authors attribute results from differing oxygen transfer rates to increased gas pressures in the RAMbio system.
DOT was a prominent factor in the Mexican study. Defined as the partial pressure of oxygen, for practical purposes this parameter is a measure of oxygen concentration or available oxygen. The authors note,
DOT differences seem to be related with an enhanced capacity of the RAMbio system and the OxyPump stoppers to replenish the gas phase with fresh air, therefore maintaining during the culture a maximum value of the oxygen partial pressure within the headspace of the flask, hence ensuring a higher OTR [oxygen transfer rate] value. (2)
Discussion
The studies discussed herein demonstrate that culture behavior is strongly influenced by both cultivation method and choice of growth medium. In the Applikon work, all cultures behaved similarly for the first five hours, and the culture medium had no significant influence on glucose consumption. However, significant differences came with the choice of culture system. OD in YPD media (yeast culture) increased slower than in LB media (bacterial culture), although higher concentrations of nutrients are made available for microorganisms in the former. Major differences in OD after five hours are explained by oxygen limitations in orbital shakers compared with the acoustic-resonance mixing system. That assumption is supported by earlier results clearly demonstrating higher levels of dissolved oxygen in a RAMbio culture than with conventional incubators (2). Applikon and its academic partners calculated that the effect improves maximum OD for RAMbio cultures by up to threefold.
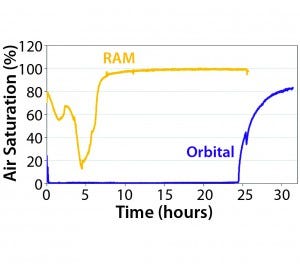
Figure 5: Dissolved oxygen profiles for Escherichia coli cultures grown with a RAMbio and orbital-shaker mixing, both using 250-mL flasks that were 20% full (4)
Resonant Acoustic mixing can be applied in small-scale systems supporting research and development of fermentation processes. In a previous study, Applikon compared RAMbio dissolved oxygen profiles with those obtained using an orbital shaker (3). Figure 5 illustrates that, by virtue of continuous oxygen replenishment, a RAMbio system provides sufficient oxygen concentrations throughout cultivation. By contrast, high oxygen demand and low replenishment kept oxygen concentrations with an orbital shaker close to zero until very late in the cultivation period. In other words, oxygen availability limited culture growth and productivity. Additional studies of oxygen transfer rates including partial pressure measurements could further improve process understanding.
Mixing Supports Growth
The oxygen transfer rates of conventional orbital shakers are insufficient for optimizing microbial fermentations. By improving oxygen transfer as much as sixfold and cell densities as much as threefold, RAMbio systems enable dense cultures in less time than orbital shakers. Optimizing cultures grown in shake flasks is of concern in many research and development settings, from basic academic experimentation to generating seed cultures for bioprocessing. Recognizing the importance of culture media optimization, many fermentation experts have switched to defined media to provide for highly reproducible cultures.
Early experiments at Applikon (and a growing body of literature) provide evidence that microbial growth depends strongly on the culture medium used. The two media under consideration in the work reported here were standard, off-the-shelf products that had not been optimized for biomass productivity or protein expression. Media notwithstanding, because oxygen is not rate-limiting in a RAMbio system, cultured microorganisms are more adept at converting glucose to biomass. In oxygen-limited orbital shaker systems, cells divert glucose to fermentation side products. Eventually acid concentrations reach critical levels, and those cultures stop growing. Exploration of optimal media and maximum biomass for new strains will be greatly enhanced with a RAMbio system by its improvement of oxygen availability in shake-flask cultures.
References
1 Biosen C-Line & Biosen S-Line. EKF Diagnostics: Cardiff, UK; www.ekfdiagnostics.com/biosen-analyzer.html.
2 Reynoso-Cereceda GI, et al. Shaken Flasks By Resonant Acoustic Mixing Versus Orbital Mixing: Mass Transfer Coefficient kLa Characterization and Escherichia coli Cultures Comparison. Biochem. Eng. J. 105(B) 2016: 379–390; doi:10.1016/j.bej.2015.10.015.
3 Application Note: Improvement of Culture Growth and Protein Expression via Use of Resonant Acoustic Mixing. Applikon Biotechnology, Inc.: Foster City, CA, 2013; www.applikonbio.com/images/download/ram/RAMbio_vs_Orbital-2013_March.pdf.
Ann D’Ambruoso is product manager for smallscale technologies at Applikon Biotechnology, 1180 Chess Dr, Foster City CA 94404; 1-650-5781396, fax 1-650-578-8836; [email protected], www.applikonbio.com.
You May Also Like






