In 2012, to mark BPI’s 10th year of publication, we launched our biennial BioProcess International Awards program. Our publication has from the start aimed to help drive advancements in this industry, not simply to report on them. We have to date (in 2012, 2014, and now 2016) recognized more than 50 people and companies that represent excellence in leadership, corporate citizenship, collaborations and partnerships, and innovative technologies and applications.
This year, our 16-member judging panel evaluated each nomination within assigned categories and scored it according to specific criteria that we provided. They assessed the same general questions as applicable to each award: applicability, innovation, results, industry impact, and sustainability.
On the evening of 5 October, at the BioProcess International Conference in Boston, publisher Brian Caine and I introduced the 24 finalists and announced the winners. We also announced the
2016 Industry Champion, Mark Petrich
(director of component enginee...
EU Workshop Report on Authorization of Advanced Therapy Medicinal Products
www.graphicstock.com
The European Medicines Agency (EMA) published a report from a multi-stakeholder expert meeting held in May exploring ways to foster the development of ATMPs in Europe and expand patients’ access to these new treatments.
ATMPs comprise gene therapies, tissue-engineered products, and somatic cell therapies. These medicines have the potential to reshape treatments of a wide range of conditions, particularly in diseases for which conventional approaches are inadequate. However, eight years since EU legislation on ATMPs went into force, only five ATMPs are currently authorized. At the same time, clinical trials investigating ATMPs are a fast-growing field of interest, underlining the need to better support innovation through a coherent and appropriate regulatory environment.
“We have organized this meeting with all relevant stakeholders to discuss concrete proposals on how we can nurture a regulatory environment tha...
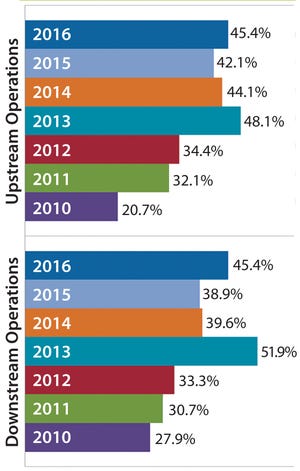
Figure 1: Percentage of biomanufacturers outsourcing at least some activity, 2010–2016
The major fluid products used in bioprocessing — culture media and buffers — are classically prepared in-house by rehydrating (dissolving and mixing) powders purchased from suppliers. Most bioprocessing facilities consider in-house preparation of these fluids to be a core bioprocessing task. However, some companies are outsourcing the work either by purchasing preprepared materials from vendors or hiring contract manufacturing organizations (CMOs) to prepare them.
Buffer fluid preparation is one area of downstream production operations that are seeing an increase in outsourced operations. These fluids are used widely in bioprocessing to equilibrate pH; condition, wash, and elute columns; and/or concentrate process streams throughout downstream purification. Buffer fluids can constitute a bottleneck in downstream processing, with required volumes that can be 10–20× the volume of bioreactors used upstream. Making these fl...
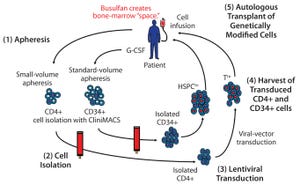
Figure 1: A cell-delivered gene therapy process begins with (
1
) apheresis, in which granulocyte colony stimulating factor (G-CSF) is administered to stimulate production of stem cells and their release into blood. Small-volume apheresis is taken for CD4+ T-cell isolation, and larger volumes are taken for CD34+ hematopoietic stem progenitor cells (HSPCs). After (
2
) a cell isolation step to purify the desired cell populations (T cells or HSPCs), purified cells are transduced with a therapeutic lentiviral vector (
3
). Following harvest of transduced T cells and HSPCs (
4
), modified cells are collected and cryopreserved. Finally (
5
), genetically modified autologous cells are transplanted back to the same patient. Busulfan chemotherapy is administered before transplantation to create “space” in the patient’s bone marrow for the therapeutically modified cells.
Cell-delivered gene therapy is making an impact on a range of diseases (
1
–
17
). To date, successful treatments have generally been in conditio...
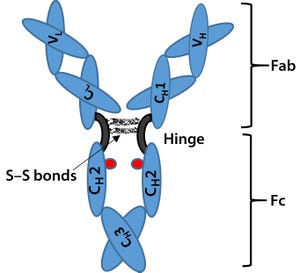
Figure 1: Immunoglobulin G (IgG) structure consists of four polypeptide chains, including two identical light chains (~25 kDa) and two identical heavy chains (~50 kDa). Each light chain consists of one constant domain (C
L
) and one variable domain (V
L
), and each heavy chain consists of three constant domains (C
H
1, C
H
2, and C
H
3) and one variable domain (V
H
). The Fab is the antigenbinding region containing hypervariable or complementarity-determining regions (CDRs), whereas the Fc region is highly conserved across molecular species. The hinge region contains two disulfide bonds and glycosylation (shown as red dots) at C
H
2 domain of Fc fragment.
Last month, Part 1 of this discussion briefly described the regulatory landscape for developing biosimilar therapeutic monoclonal antibodies (TMAbs). We identified certain specific structural components of TMAb drug substances that warrant particular attention because alterations to them are likely to affect therapeutic safety and effectiveness. Now we c...
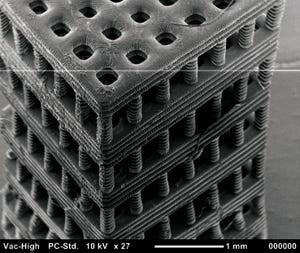
Figure 4: Tissue scaffold fabricated with stereolithography from a biocompatible and biodegradable polymer. Chartrain NA, et al. Microstereolithography of Tissue Scaffolds Using a Biodegradable Photocurable Polyester.
International Solid Freeform Fabrication Symposium
: Austin, TX, 2015 (in press).
Commonly referred to as three-dimensional (3D) printing,
additive manufacturing
encompasses a set of technologies that fabricate objects in an additive way, layer by layer, rather than conventional means of fabrications that generally subtract unwanted material from a larger block. Precise control over material placement allows 3D printing to fabricate objects that otherwise would not be manufacturable. Although many of these technologies have been around for two or three decades, recently they have received a significant amount of attention from industry, academia, and the general public. 3D printed components are now fabricated as end-user parts in the automotive, biomedical, and aerospace industries. Howe...
Conjugated protein biotherapeutics such as PEGylated proteins (with polyethylene glycol), antibody–drug conjugates (ADCs), and protein–haptens often present unique analytical challenges related to characterizing the conjugation aspect of their manufacturing processes. Analytical characterization of this class of proteins requires knowledge of the sites of conjugation, the degree of conjugation, and the drug-to-protein ratio.
Here we present case studies in development of reliable methods based on mass spectrometry (MS) to characterize a protein–hapten drug substance during late-phase process validation. This protein is modified by succinylation to enable conjugation with an aminated hapten.
The Challenge
Haptens are small molecules and usually by themselves nonimmunogenic. They must be attached to a macromolecule (e.g., protein, peptide, or synthetic polyamino acid) to solicit a hapten-specific immune response. In our case study, a client required an accelerated 16-month program for technology transfer an...
GRAPHIC STOCK (WWW.GRAPHICSTOCK.COM)
As the biologics (and now biosimilar) markets continue to grow, pressure increases on biomanufacturers to reduce cost of goods sold (CoGS). One way they can reduce cost is by increasing protein productivity in terms of protein titer per volume of culture. Media optimization is a key strategy for increasing protein productivity. In the past few decades, average titers across the industry have increased greatly — from <0.5 g/L in the 1980s to >3 g/L today, and it is not uncommon now to see titers of ~5 g/L (
1
). Despite those improvements in protein output, the drive to continue optimizing processes remains, not only to further increase expression titers, but also to improve protein quality.
As biomanufacturers continue to improve their production processes for increased protein titers, they also must ensure that protein stability, solubility, and efficacy are not negatively affected. Other challenges to increasing yield include scalability, downstream process efficienc...
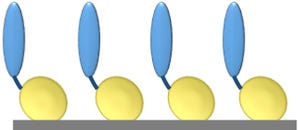
Figure 1: Core protein engineering technology; scaffold protein is shown in yellow, and the fused protein of interest (e.g., cell-adhesion protein, enzyme, or single-chain antibody molecule) is shown in blue.
Production of proteins for pharmaceutical use is a complex, multistep process that requires technologies for purifying such molecules from highly complex biological mixtures. It also calls for reliable, cost-effective, high-throughput analytical techniques to determine protein quality and functionality to ensure the safety and efficacy of end-products. Mistakes in product development and manufacturing not only are immensely costly, but they can also put patients at risk.
Many well-established processes and analytical tools are available for use in manufacturing antibody drugs (e.g., protein A resins for purification). But next generation drug products — including antibody-drug conjugates (ADCs), nonantibody protein drugs, and therapeutic cells — bring fresh challenges in biomanufacturing. Such produc...
with Paul Jorjorian
In BPI’s 5 October 2016 webcast, Paul Jorjorian (director of global technology transfer at Patheon) discussed his top five considerations for companies outsourcing to a biomanufacturing partner.
Jorjorian’s Presentation
Selecting a contract and development manufacturing organization (CDMO) is an important decision for many biopharmaceutical companies. Beyond time and cost, here are some other criteria to consider.
The number-one issue is
quality
. Is your company’s definition of quality the same as a CDMOs — and if not, how do those definitions differ? Here are three main discussion points: understanding how a CDMO addresses deviations; creating a quality agreement; and determining who is responsible for making, reviewing, and approving required documents.
The second consideration is
technical expertise
. Does a CDMO’s technical expertise fit your needs? Examples include experience with the different phases of drug development, process characterization studies, recombinant or fusion ...
with Chris Rombach
Open-suite biomanufacturing is gaining popularity. But it leaves surfaces open to contamination, workers at risk for exposure to airborne particulates, and processes more exposed to product loss due to waste or spillage. Liquid-handling systems are often adapted for dry-powder applications, but flow rates, handling characteristics, and dispensing volumes differ dramatically. ILC Dover’s purpose-built, single-use EZ BioPac system is designed specifically to contain and release dry powders. With single-use bags engineered for the task, it can deliver several advantages: reducing spillage, waste, and processing time by filling quickly and precisely, sealing completely and dispensing swiftly and cleanly, leaving no residue inside, and eliminating unwieldy barrels and containers.
In an “Ask the Expert” webinar on 31 August 2016, Chris Rombach (ILC Dover’s director of global strategic accounts) described how the system works and how its critical components are designed. He supported its speci...
with Vincent Monchois
In our September webcast, Vincent Monchois (biopharma strategic project director at Novasep) discussed continuous chromatography using BioSC technology. He included regulatory considerations and the challenges of viral clearance that arise when companies switch from batch to continuous processes.
Monchois’s Presentation
Users can apply BioSC multicolumn, continuous chromatography to standard capture steps such as ion-exchange or protein A chromatography. The main advantages are continuous operation and full access to the total static capacity of a resin. Other advantages include reduction of production costs (in terms of resins, buffers, volume, and footprint), a streamlined process, and increased productivity. Eluted product quality is the same as that from batch processing.
This technology works by repeating identical cycles, allowing for all columns to run an entire process. The key is to operate at a steady state, ensuring no change in critical attributes.
A
batch
is defined as...
Stefan Schlack (Photo by Connect Upstream)
The biopharmaceutical industry is enjoying considerable success. Its products account for about a fifth of world pharmaceutical revenues, which are growing at twice the pace of those generated by most traditional chemically synthesized drugs. Biopharmaceuticals populate the list of best-selling drugs, and a number have achieved blockbuster status. Biotechnology stocks have outperformed the general market as investment has flowed into the industry.
As with other highly profitable markets, the market for biopharmaceuticals has become increasingly competitive. Reflecting this fact, in September 2015 Sandoz launched Zarxio (filgrastim-sndz), the first biosimilar product approved by the US Food and Drug Administration for use in the United States (1). Competition from a tide of biosimilar products is on the way, forcing biomanufacturers to consider how best to optimize their operations for maximum efficiency and lowest cost. The following figures show analysis of 2016...