Biosimilar Therapeutic Monoclonal Antibodies: Gaps in Science Limit Development of an Industry Standard for Their Regulatory Approval, Part 2Biosimilar Therapeutic Monoclonal Antibodies: Gaps in Science Limit Development of an Industry Standard for Their Regulatory Approval, Part 2
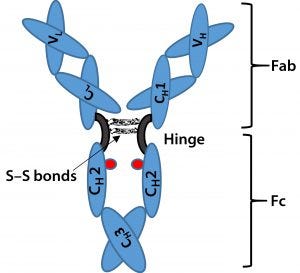
Figure 1: Immunoglobulin G (IgG) structure consists of four polypeptide chains, including two identical light chains (~25 kDa) and two identical heavy chains (~50 kDa). Each light chain consists of one constant domain (CL) and one variable domain (VL), and each heavy chain consists of three constant domains (CH1, CH2, and CH3) and one variable domain (VH). The Fab is the antigenbinding region containing hypervariable or complementarity-determining regions (CDRs), whereas the Fc region is highly conserved across molecular species. The hinge region contains two disulfide bonds and glycosylation (shown as red dots) at CH2 domain of Fc fragment.
Last month, Part 1 of this discussion briefly described the regulatory landscape for developing biosimilar therapeutic monoclonal antibodies (TMAbs). We identified certain specific structural components of TMAb drug substances that warrant particular attention because alterations to them are likely to affect therapeutic safety and effectiveness. Now we conclude by considering whether studies of reference materials can further the development of analytical industry standards to ensure comparability of putative biosimilar TMAbs with innovator TMAbs. We suggest that the time is right to tie analytical industry standards and manufacturing controls with specific reductions in preclinical and clinical studies for regulatory approval of certain biosimilar TMAbs.
Drug Product: Aggregation and Excipients
Aggregated TMAbs as an impurity can cause adverse toxicological and immunological responses or may substantially reduce drug efficacy. So aggregation needs to be characterized at different stages in a biopharmaceutical product’s lifecycle. Changes in cell culture processes (e.g., fermentation media, temperature, and pH) have been shown to affect TMAb aggregation. Aggregates also can be formed during purification, formulation, and product filling (55, 56). Other factors, such as freeze–thaw cycles, protein concentration, and chemical modifications also can affect the formation of protein aggregates (57). Moreover, the scrambling of disulfide bonds caused by alterations in chemical reduction–oxidation can lead to aggregation.
A number of techniques are used to analyze protein aggregation: analytical ultracentrifugation (AUC), field-flow fractionation (FFF), size-exclusion chromatography (SEC), dynamic light scattering (DLS) (58), and electrospray injection (ESI) time of flight (TOF) mass spectrometry (MS) in combination with high-performance SEC (59). Recent studies have indicated that electrospray–differential mobility analysis (ES-DMA) can be an orthogonal technique to characterize protein aggregates (60). Advantages, limitations, and specifics of each technique have been extensively studied and reviewed (61–63).
Excipients such as arginine and sugars are used to suppress aggregation during purification and formulation processes (64–66). But despite their use, aggregation remains a concern. Particles vary in size, reaching even tens of microns (58, 61). Although sensitive and robust analytical tools can be used to assess these aggregates (58), the challenge remains to fully analyze protein aggregates when sizes span up to six orders of magnitude. An understanding of the clinical impact of unwanted aggregate particles on product safety and efficacy generally is unavailable, so predicting biosimilarity without detailed knowledge of an innovator’s manufacturing processes appears impractical at this time.
Reference Abs: Verifying Biosimilarity
An innovator TMAb product’s approved manufacturing process and related data describing drug product variability are all proprietary. One way to establish comparability of laboratory findings between different industry sponsors is to validate their test methods using the same substrate (32). For this reason, independent sponsors attempting to develop biosimilar TMAbs must purchase multiple lots of innovator product to learn what level of variability might be acceptable (31). Competition with the originator is disadvantaged when developing biosimilar TMAbs because analytical methodologies also can differ between industry sponsors. That makes it difficult for regulatory agencies to determine whether the competitor product meets the manufacturing standards of the innovator.
One possibility that has been suggested to verify chemical comparability between TMAbs materials is to calibrate analytical methods against a widely available reference. Fundamental to establishing equivalence is validation of an analytical method(s) to be used. In that light, the National Institute of Standards and Technology (NIST) has been studying a humanized IgG1κ molecule of G1m(3)Km3 allotype as a possible reference standard (67). The so called NISTMAb is produced using mammalian cell culture and formulated in 12.5 mM l-histidine or 12.5 mM l-histidine HCl monohydrate (pH 6.0) (68). The NISTMAb is intended to serve as a public reference material that also can be used to ensure performance of analytical and biophysical technologies (e.g., instrument performance and potency standards for method qualification).
NIST’s reference materials provide a common basis for qualifying methods and are made publicly available (without intellectual property concerns). Analytical and biophysical methods for biosimilar characterization can differ greatly from methods used with innovator products. So the extensive characterization data of TMAb reference material using state-of-the-art techniques also should help companies determine the dynamic range, detection limits, linearity, and precision of advanced technologies. Use of publicly available reference materials and a database of test results from evaluation of those materials could inform future development of industry-wide standards for measurement of TMAb heterogeneity, product quality, and test method qualification.
That said, a number of additional reference TMAbs may be needed to cover the diversity of biosimilar targets. For example, CHO cells will glycosylate a given TMAb differently than will a murine cell line such as NS0 or SP2/0. The current NISTMAb was produced in NS0; it could be expressed in CHO cells and analyzed to elucidate and highlight these differences in glycan and other posttranslational modifications using NIST validated methods. Other next-generation TMAb isotypes such as that of the humanized hybrid IgG2/4 eculizumab (Alexion’s Soliris product) (69) could warrant development of a reference standard to capture unique posttranslational variations such as disulfide isoforms.
The implication is that once certain analytical technologies have been accepted by regulatory agencies for use with TMAbs, then chemical comparability can be established by showing analytical accuracy between innovator and biosimilar pharmaceutical manufacturers against a standard reference Ab material. That is particularly attractive for cross-class quality attributes. However, measures of product-specific critical quality attributes (CQA) may require optimization for accommodating product variability, potentially bracketing predictability of the “reference” Abs with respect to clinical safety and efficacy. Note that the active pharmaceutical substance (free of impurities or excipients) must be extracted from a final drug product to be tested for potency and CQA (e.g., glycosylation analysis using HPLC and CE). The extraction method can affect the quality of the reference product, making it less reliable for use in comparability tests (70). Nonetheless, if the CQA profile of a biosimilar TMAb has been carefully related to clinical performance, then a quality-by-design (QbD) risk-based approach as described in the FDA’s process analytical technology (PAT) guidance for industry would be applicable for biosimilar development (71).
In addition to an intact-MAb reference standard such as that under development by NIST, the industry greatly needs a library of glycan quantification standards. Current commercially available reference products are used by quality analysts to establish retention time and migration in HPLC and CE assays, respectively, but they do not allow for quantitation. Also, the available standards are typically prestained with established tags such as 2-aminobenzoic acid (2-AA), 2-aminobenzaminde (2-AB), or 9-aminopyrene-1,4,6-trisulfonic acid (ATPS). Unstained standards may or may not be amenable to novel or improved tags such as procainamide (72) and Waters RapiFluor-MS reagent (73).
Even for innovators, maintenance of a CQA profile could be improved by development of “standardized” manufacturing methodologies (74). Production concepts developed previously for use in small-molecule pharmaceutical manufacturing and in other bioprocess industries (e.g., fuels and fermented products) could be applied to the TMAb manufacturing industry and better enable uniform product output. Continuous manufacturing processes minimize perturbations relative to batch or fed-batch processes (74, 75). They also enable more reliable real-time process optimization and control.
One challenge for the latter is a general lack of “standardized” PATs that enable rapid measure of product quality and yield. Continued development is needed. For example, as noted above few techniques are available for rapid assessment of Ab aggregation. Although product heterogeneity (including glycosylation state) is evaluated as a CQA, few if any methods can measure production process heterogeneity — and we are aware of no linkage to it. Further, there are no “standardized” production cell lines that could enable cross-class manufacturability. Such innovations would be applicable to both innovator and biosimilar TMAbs, probably speeding regulatory approval for both as well.
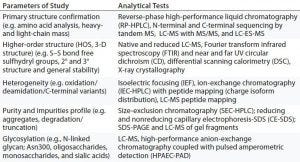
Table 3: Analytical data for physicochemical characterization and comparison of Remicade (innovator) and Inflectra (biosimilar) infliximab
Case Study: Approval of Biosimilar Infliximab in Europe
In 2016, Inflectra (infliximab from Celltrion) was the first biosimilar TMAb approved by the US FDA (15). Both it and Remsima (infliximab from Hospira) had been approved in Europe as biosimilars (2013) to the drug substance of Remicade (infliximab from Janssen). The biosimilar infliximabs are chimeric anti-TNFα MAbs of the IgG1k subclass expressed in murine hybridoma cells. They are highly similar to Remicade chemically, in vitro, and in preclinical animal models with the exceptions of afucosylated glycans (74). Nonclinical comparability characterization included LC-MS, high-performance anion-exchange chromatography coupled with pulsed amperometric detection (HPAEC-PAD), FT-IR spectroscopy, near and far UV CD, DSC, and X-ray crystallography (Table 3).
Minor differences were detected in purity (with slightly higher aggregate levels occurring in biosimilar infliximab), C-terminal lysine variability, and concentration of intact IgG, but the TNFα binding affinity and potency were not affected. So biosimilar infliximab was comparable to the original in terms of binding to Fc receptors and C1q, and it comparably suppressed cytokine secretion. However, biosimilar infliximab showed significantly reduced binding to FcγRIIIa/b receptor and decreased antibody-dependent, cell-mediated cytotoxicity (ADCC) activity when tested with enriched NK cells of healthy donors and patients suffering from Crohn’s disease.
The binding affinity and ADCC were comparable for biosimilar and original infliximab in physiologically relevant conditions — e.g., in human peripheral blood mononucleated cells (PBMC) from Crohn’s disease patients and healthy donors, a wound healing model, or in whole blood. Furthermore, comparative repeat-dose rat toxicity and pharmacology studies also gave similar findings despite low binding affinity with FcγRIIIa. Apparently, the binding variability came from the different cell lines producing the TMAbs in addition to minor manufacturing differences rather than analytical errors.

Table 4: Comparing nonclinical and clinical development of Remicade (innovator) and Inflectra (biosimilar) infliximab products for regulatory approval in Europe
The regulatory burden is decreased for clinical data (Table 4) because of close comparability in product quality and manufacturing methods between biosimilar and original infliximab. In a randomized, double-blinded pharmacokinetic study (CT-P13 1.1) among 250 patients with ankylosing spondylitis (AS), biosimilar and Remicade infliximabs exhibited bioequivalence (77). Comparable pharmacokinetics were reported for geometric means in the area under the curve (AUC) of concentration over time curve and for maximum serum concentration (Cmax). Immunogenicity and safety profiles in that pharmacokinetic study were reassuring because similar proportions of patients developed antiinfliximab antibodies (27.4% and 22.5% of subjects, respectively) and adverse events (64.8% and 63.9% of subjects, respectively).
Only a single pivotal trial (CTP13 3.1) of efficacy and safety was conducted for EU approval. In this study of 606 adult patients with active rheumatoid arthritis (RA), clinical outcome findings were statistically comparable. For example, at week 30, efficacy evidenced by ACR20 (20% improvement, as defined by the American College of Rheumatology) responses were 60.9% for biosimilar infliximab and 58.6% for Remicade infliximab. The adverse event rates were reported at 35.2% and 35.9% of subjects, respectively. Moreover, the pivotal-study patients developed antidrug antibodies at similar rates for biosimilar and Remicade infliximabs (48.4% and 48.2%), respectively.
Biosimilar infliximab eventually was approved on the EU market for other approved Remicade indications, namely psoriatic arthritis, psoriasis, and inflammatory bowel diseases (IBDs) such as Crohn’s disease and ulcerative colitis, but without conducting additional clinical trials (75, 76). The EMA justified its extrapolation of approval for other clinical indications despite ADCC differences between innovator and biosimilar TMAbs because ADCC was not believed to be the primary mechanism of action for efficacy in each of those clinical conditions. Canadian regulators did not agree with their European counterparts, however, so an IBD indication was not approved there.
In Search of a Standard
The FDA’s biosimilars guidance describes elements of a development pathway for the US market (22). However, uncertainties remain regarding the specific type and level of scientific evidence (analytical, preclinical, and clinical studies) needed for a biosimilar product to reduce its regulatory and scientific burden relative to an innovator product. To identify the structure–function relationship of a MAb, complete understanding of the product and its quality attributes is required. Given the medical and economic value of TMAb products — stemming from their complex structure and complicated manufacturing process — a class-specific guidance for creating a regulatory pathway specifically tailored for TMAbs should address the issues raised by their unique development challenges.
The integration of QbD strategies into manufacturing can help facilitate process design and final-product quality and consistency. Acceptance of TMAb reference materials such as those from NIST should address key challenges, including method qualification for measuring aggregates and variations in posttranslational modification. Furthermore, evaluation of reference CQAs relative to the standard’s manufacturing conditions (which like its HOS data should be publicly available) will provide needed datasets for establishing predictions of product quality. We do note, however, that a single reference standard is not expected to establish cross-class CQAs: Quality attributes of TMAbs are likely to require evaluation specific to each product and host cell line.
At this time, the policy for regulating biosimilar pharmaceuticals outlined in the Public Health Service Act (7), added to by the Affordable Care Act, appears to be ahead of the science relating structure to function for TMAbs. Developers of biosimilar TMAbs must continue to study proprietary samples of innovator TMAbs to fully validate their own manufacturing process controls and analytical methods used for assaying quality. However, we believe that regulatory agencies can take steps to clarify their requirements and lower the burden to developers of biosimilar TMAbs. Those steps would lead incrementally to broader industry standards for manufacturing, laboratory, and nonclinical testing — thereby reducing reliance on complex clinical studies to provide requisite evidence of safety and effectiveness.
The “Recommendations” box describes our recommendations that could facilitate creation of standards tailored to the unique issues raised by the TMAb class of biological products, thus streamlining the approval process.
Recommendations |
|---|
Analytical assays and manufacturing procedures for TMAbs should be developed for validation and standardization to narrow critical quality attributes (CQAs) across the product class and streamline regulatory review. This includes creation of new methodologies and techniques that would create more uniform products (e.g., continuous processes, innovative process analytical technolgies, and model-based control methodologies). |
Regulatory bodies should evaluate use of TMAb reference materials including quantitative glycan standards for calibration of instrumentation and analytical methodologies to enable developers of biosimilar TMAbs to reduce the number of proprietary lots needed for study from innovators. However, a certain number of reference lots will be needed to establish a window/range of each CQA based on innovator lot-to-lot variability. |
Regulatory agencies should define specification limits for biosimilarity by assigning an acceptable range for each parameter (e.g., glycosylation, disulfide structure, charge variants, size variants) within which biosimilars must fall in comparison to innovator reference lots, creating product class-wide acceptance criteria. |
Regulatory agencies should provide more explicit guidance for validation of structure–function relationships (e.g., IgG-Fc glycoform and functional activity such as ADCC) with respect to safety and efficacy for reducing inconsistencies among regulatory bodies that review biosimilar TMAbs. |
Acknowledgments
This work was supported with funds and a fellowship award from the Global Biological Standards Institute (GBSI). We thank Dr. Anthony Mire-Sluis at Amgen Inc. (California) and Dr. Hans Ebbers at the Utrecht Institute for Pharmaceutical Sciences (The Netherlands) for their feedback and critical review. We are grateful to University of Maryland and GBSI staff for their support of this project. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agency.
References (from Part 1)
7 Public Law 111-148, Section 1695. Biologics Price Competition and Innovation Act. Provisions of the Patient Protection and Affordable Care Act. 110th Congress: 23 March 2010.
15 Chowdhury BA. BLA Approval 125544. United States Food and Drug Administration: Rockville, MD, April 2016.
22 CDER/CBER. Guidance for Industry: Biosimilars — Questions and Answers Regarding Implementation of the Biologics Price Competition and Innovation Act of 2009. US Food and Drug Administration: Rockville, MD, April 2015.
31 McCamish M, Woollett G. Worldwide Experience with Biosimilar Development. MAbs 3(2) 2011: 209–217.
32 McCamish M, Woollett G. The State of the Art in the Development of Biosimilars. Clin. Pharmacol. Ther. 91(3) 2012: 405–417.
References (Part 2)
55 Vazquez-Rey M, Lang DA. Aggregates in Monoclonal Antibody Manufacturing Processes. Biotechnol. Bioeng. 108(7) 2011: 1494–1508.
56 Bahrami A, et al. Prevention of Human Granulocyte Colony-Stimulating Factor Protein Aggregation in Recombinant Pichia pastoris Fed-Batch Fermentation Using Additives. Biotechnol. Appl. Biochem. 52(Pt 2) 2009: 141–148.
57 Mahler HC, et al. Protein Aggregation: Pathways, Induction Factors and Analysis. J. Pharm. Sci. 98(9) 2009: 2909–2934.
58 den Engelsman J, et al. Strategies for the Assessment of Protein Aggregates in Pharmaceutical Biotech Product Development. Pharm. Res. 28(4) 2011: 920–933.
59 Kukrer B, et al. Mass Spectrometric Analysis of Intact Human Monoclonal Antibody Aggregates Fractionated By Size-Exclusion Chromatography. Pharm. Res. 27(10) 2010: 2197–2204.
60 Guha S, et al. Electrospray-Differential Mobility Analysis As an Orthogonal Tool to Size-Exclusion Chromatography for Characterization of Protein Aggregates. J. Pharm. Sci. 101(6) 2012: 1985–1994.
61 Demeule B, et al. Characterization of Particles in Protein Solutions: Reaching the Limits of Current Technologies. AAPS J. 12(4) 2010: 708–715.
62 Gabrielson JP, et al. Common Excipients Impair Detection of Protein Aggregates During Sedimentation Velocity Analytical Ultracentrifugation. J. Pharm. Sci. 98(1) 2009: 50–62.
63 Philo JS. A Critical Review of Methods for Size Characterization of Non-Particulate Protein Aggregates. Curr. Pharm. Biotechnol. 10(4) 2009: 359–372.
64 Shah D, et al. Effects of Arginine on Heat-Induced Aggregation of Concentrated Protein Solutions. Biotechnol. Prog. 27(2) 2011: 513–520.
65 Cleland JL, et al. A Specific Molar Ratio of Stabilizer to Protein Is Required for Storage Stability of a Lyophilized Monoclonal Antibody. J. Pharm. Sci. 90(3) 2001: 310–321.
66 Kheddo P, et al. The Effect of Arginine Glutamate on the Stability of Monoclonal Antibodies in Solution. Int. J. Pharm. 473(1–2) 2014: 126–133.
67 Schiel JE, Davis DL, Borisov OV. State-of-the-Art and Emerging Technologies for Therapeutic Monoclonal Antibody Characterization, Volume 2: Biopharmaceutical Characterization — The NISTmAB Case Study. American Chemical Society Symposium: Washington, DC, October 2015.
68 Schiel JE, Davis DL, Borisov OV. State-of-the-Art and Emerging Technologies for Therapeutic Monoclonal Antibody Characterization, Volume 1: Monoclonal Antibody Therapeutics — Structure, Function, and Regulatory Space. ACS American Chemical Society Symposium: Washington, DC, 2014.
69 Rother RP, et al. Discovery and Development of the Complement Inhibitor Eculizumab for the Treatment of Paroxysmal Nocturnal Hemoglobinuria. Nat. Biotechnol. 25(11) 2007: 1256–1264.
70 Liu C, et al. Assessment of the Quality and Structural Integrity of a Complex Glycoprotein Mixture Following Extraction from the Formulated Biopharmaceutical Drug Product. J. Pharm. Biomed. Anal. 54(1) 2011: 27–36.
71 CDER/CVM/ORA. Guidance for Industry: PAT — A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance. US Food and Drug Administration: Rockville, MD, September 2004.
72 Kozak RP, et al. Procainamide Labelling As Part of a Flexible Glycoprofiling System for Monitoring of Gal-a1-3Gal Related Glycosylation Critical Quality Attributes (GCQAs) of Monoclonal Antibody (MAb) Therapeutics Throughout the Product Life Cycle (poster). WCBP 2016: 20th Symposium on the Interface of Regulatory and Analytical Sciences for Biotechnology Health Products, Washington DC, January 2016.
73 White Paper: Best Practices in the Analysis of RAPIFluor-MS Labeled Glycans Using the Acquity QDa Detector (Performance Model). Waters Corp.: Milford, MA, March 2016.
74 Croughan MS, Konstantinov KB, Cooney CL. The Future of Industrial Bioprocessing: Batch or Continuous? Biotechnol. Bioeng. 112(4) 2015: 648–651.
75 Konstantinov KB, Cooney CL. White Paper on Continuous Bioprocessing: May 20–21, 2014 Continuous Manufacturing Symposium. J. Pharm. Sci. 104(3) 2015: 813–820.
76 Ebbers HC, Biosimilars: In Support of Extrapolation of Indications. J. Crohns Colitis 8(5) 2014: 431–435.
Simran J. Kaur is an ORISE research fellow at the US Food and Drug Administration; Darryl Sampey is president and CEO of BioFactura, Inc. (Frederick MD); Lester W. Schultheis is director of the Maryland Center of Excellence in Regulatory Science and Innovation (M-CERSI), part of the Fischell Department of Bioengineering at the University of Maryland (College Park); and Leonard P. Freedman is president of the Global Biological Standards Institute (Washington, DC). Corresponding author William E. Bentley is a principal investigator at the University of Maryland (Rockville) Institute for Bioscience and Biotechnology Research, and a distinguished professor at M-CERSI, 2330 Jeong H. Kim Engineering Building, Fischell Department of Bioengineering, University of Maryland, College Park 20742; 1-301-405-4321, fax 1-301-405-9953; [email protected].
You May Also Like






