November-December 2018
WWW.COPPERHOUNDPICTURES.COM
The word
innovation
carries with it connotations of both newness and change. Funny thing about that: Changing conditions require adaptation and originality in response to them — which often causes more change that will in turn require further innovation in response. In an industry based on science, this can (and should) be a neverending cycle either of playing catch-up — or of continually pushing the envelope.
We hear a lot of talk about innovation in the biopharmaceutical industry these days. Some people lament the absence or inadequacy of it; others want to tout nearly every small improvement as “innovative.” Although the latter may be prone to marketing hype, I think that folks in the former camp may not be seeing the forest past the trees immediately surrounding them. From my journalistic perspective as an informed observer on the outside looking in, that forest seems both healthy and diverse — if not quite as dazzling as public and investor relations groups might suggest...
Launched in June 2018, the
BioProcess Insider
digital information portal delivers the latest financial and business news and expert insider views influencing the commercialization of biopharmaceuticals. Here are just a few recent stories edited for our space limitations in print. For more discussion and in-depth analysis, check out the website at
www.bioprocessinsider.com
. Every edition provides expert and insider perspectives on current financial movements and deal-making; the newest technology purchases and capacity investments; regulations affecting the bioprocessing sector; global market actions and reactions; and industry trends.
Janssen Opens HIV Vaccine Plant
Janssen Pharmaceuticals, a subsidiary of Johnson & Johnson, has opened a new single-use biomanufacturing facility to support commercialization of a preventative human immunodeficiency virus (HIV) vaccine candidate in development. Representing an investment of €72 million ($82 million), the multipurpose vaccine-production facility was inaug...
A Multistep Research Protocol to Develop and Implement Validated Guidelines for CMO RFI and RFP Processes: Biopharmaceutical Vendor Evaluation and Selection Minimum Standards (BioVesel)A Multistep Research Protocol to Develop and Implement Validated Guidelines for CMO RFI and RFP Processes: Biopharmaceutical Vendor Evaluation and Selection Minimum Standards (BioVesel)
WWW.ISTOCKPHOTO.COM
Pursuant to the proposal for validated minimum standards for biopharmaceutical contract manufacturing organization (CMO) request-for-information (RFI) and request-for-proposal (RFP) processes (
biopharmaceutical vendor evaluation and selection minimum standards
, BioVesel) (
1
), we propose herein a multistep research protocol to develop and implement the BioVesel standards. This proposal is intended as a basis for discussion among mulitple stakeholders. Detailed research protocols for each proposed stage in the development and implementation of BioVesel will be drafted and published separately. The context of the proposed research is an exercise to gather and synthesize the opinions of stakeholders involved in the evaluation, acquisition, provision, and oversight of biopharmaceutical CMO services (Table 1).
Table 1: Overview of stakeholders engaged in contract manufacturing organization (CMO) service evaluation, acquisition, provision, and oversight
Table 1 shows the many stakeholders...
HTTPS://STOCK.ADOBE.COM
On Thursday 6 September 2018 at the annual BioProcess International Conference in Boston, the first “Technology Round Robin Featuring Six Innovative Bioprocess Technologies” was presented in an interactive session with attendees as active participants, asking questions and engaging in conversation with the six featured entrepreneurs. Detailed below, this session was a culmination of several steps in an overall strategy for some of the companies participating. To fully appreciate the launch of new technologies into the bioprocess arena, you first must understand the challenges faced by such early stage suppliers and what we can do to support technology development and innovation in support of the biopharmaceutical industry.
Figure 1: The “valley of death” in biopharmaceutical drug development
Drug development has an identified “valley of death” that comes when the gulf widens between finding a promising new therapy and moving to the necessary clinical trials that can demonstrate its...
STOCK.ADOBE.COM
In earlier issues of BPI we published a few “Elucidation” closers that we called “Defining Moments.” Since then, we have tried to distinguish key confusable terms from one another. Those presented (and sometimes “elucidated”) have been
analytical
and
bioanalytical
,
spectroscopy
and
spectrometry
, and
biosimilars
and
biobetters
. They are just a few of the many confusable terms in the biopharmaceutical industry. For example, when someone says “drug delivery,” a formulator will think of a syringe or transdermal patch, but a logistics expert will think of packaging or a truck.
Figure 1: Most survey participants reside and work in Europe or North America.
The term
biopharmaceutical
itself was coined more to distinguish that category of recombinant large biomolecules from what was then the more familiar “classical” or small-molecule drugs — those chemically synthesized drug substances that can be delivered mainly through oral formulations. In fact, the word biopharmaceutical was coi...
Figure 1: The product cycle model of innovation (
1
)
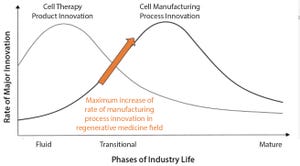
Most commercial biopharmaceuticals originated from academic research laboratories and start-up development laboratories. Despite such products having differences in modalities and targeted disease indications, and whether their target patient populations are relatively small or approaching blockbuster status, at a key point in development, biopharmaceutical production must scale up from laboratory to commercial production. That movement from research to development and then to manufacturing forces attention on economics and speed to market, and it drives innovative approaches to producing biopharmaceutical cell compositions at commercially relevant scales and at cost structures that support business models.
Regenerative medicines, whether for cell therapies or biofabricated tissues, now face that same transition. Bioprocess developers require a steady supply of consistent, highly functional, highly viable cells at large volumes for both product and pro...
In commercial-scale biopharmaceutical manufacturing, downstream chromatography steps are still a bottleneck and contribute to significant operational costs (
1
,
2
). Some of those costs are inherent (e.g., resins, large buffer quantities, and cleaning) whereas others are avoidable (e.g., product loss due to rejected lots or deviations that result in production downtime). Maintaining efficient and robust chromatography process performance is therefore critical for minimizing operating costs. To do so, we introduce a simple and one-point multiparameter technique (SOP-MPT) for monitoring chromatographic process performance.
Traditionally, chromatography process performance has been monitored by analytical assays (e.g., those measuring yield, impurities, and molecular integrity) along with visual review of chromatographic profiles by trained personnel. However, such techniques have limitations. Analytical assays are performed for each production lot but not necessarily at each process step, thus creating ga...
FortéBio’s Octet instruments are an ideal replacement for ELISA, HPLC, and SPR techniques in quantification of antibodies and recombinant proteins and in testing product potency for lot release. Bio-Layer Interferometry (BLI) technology monitors biomolecular interactions in real time to determine affinity, kinetics, and concentration. The plate-based, microfluidics-free format offers users several distinct advantages over other technologies. BLI-based systems can achieve higher throughput, with the flexibility to measure two to 96 samples simultaneously. Lower maintenance requirements and increased ease-of-use further shorten the time it take to get results. Increased system flexibility — including an ability to analyze unpurified samples and tolerate a broad range of conditions — is another time-saving hallmark. With additional tools to enable integration into regulated environments, the Octet systems can be instituted across biologics development.
Figure 1: Overlay of several replicates of FcRn–IgG inte...
Veterans of the US military still struggle to access healthcare despite the 2014 congressional passing of the Veteran’s Choice Program (VCP), a US$10 billion-dollar “fix” that allows qualifying veterans to see community physicians who have contracted with the Department of Veterans Affairs (VA) to provide care. Veterans who enrolled in VCP to avoid long wait times at department medical facilities still have faced month-long delays before seeing a doctor, according to a
2018 GAO report
. Investigators have found that the VA protocol for scheduling VCP appointments caused veterans to wait up to 81 days for treatment and an average 51 days to receive care, despite the VA’s own standard to administer care within 30 days of a patient’s request.
One key factor in the failed implementation of VCP could be the excessive delay in physician reimbursement that ultimately is driving away community provider participation in the program. Hospitals, clinics, and doctors across the country have complained about not gett...
On 24 October 2018, BPI presented a free “Ask the Expert” webinar with Abel Hastings, director of process sciences at Fujifilm Diosynth Biotechologies. He discussed the use of systematic tools to expedite process characterization and maximize reliability of process validation campaigns.
Hastings’s Presentation
As a project moves from clinical manufacturing toward process validation — and ultimately toward preapproval inspections — project timelines can become hypervisible at all levels of an organization. Missteps can be costly. The commercial viability of a program can be at stake.
It’s important to maintain focus. Fujifilm Diosynth Biotechologies (FDB) uses systematic tools to strike a balance between science and speed to product launch. Science is paramount, with risk-based focus on activities that are of greatest value to the project. The company has built a suite of such tools for process characterization and validation called Risk Assessment Process Template Application (RAPTA). It helps drive a sys...
On 18 October 2018, BPI presented a free “Ask the Expert” webinar with Jamie Freeman, a bioproduction product manager at Horizon Discovery. His company is developing a whole-genome screen using clustered regularly interspaced short palindromic repeats (CRISPR) for improving the capacity of Chinese hamster ovary (CHO) cells in biomanufacturing.
Freeman’s Presentation
Freeman described the screening approach that Horizon developed to improve its own CHO cells. It also may be used to improve other such cell lines for biomanufacturing. Founded 11 years ago based on cell-line engineering using recombinant adenoassociated virus (rAAV), this company now works on all aspects of genome engineering and its applications to the use of cell lines in drug discovery, development, and manufacture.
Regulator-approved cell lines using the glutamine synthetase (GS) selection system have provided established mechanisms for generating clones at commercially viable and relevant levels. But users have been frustrated with licen...
On 20 September 2018, Jiali Liao (principal scientist in process chromatography R&D at Bio-Rad Laboratories) led a BPI “Ask the Expert” webinar introducing the high-performance, high-capacity Nuvia HP-Q Anion Exchange Resin, which can be used for purifying large biomolecules.
Liao’s Presentation
Purifying large biomolecules — e.g., plasma proteins, immunoglobulins, viruses, virus-like particles (VLPs), and PEGylated proteins — can be difficult. Their size makes for slow diffusion through the pores of traditional chromatography resins and poor mass-transfer kinetics, which decreases binding capacity. Many resins currently on the market thus are limited in their ability to bind large molecules. For some, the pore size is too small; others have too small a binding area.
Nuvia HP-Q Resin is based on Bio-Rad’s established highly porous UNOsphere technology. Particle size of the rigid base beads is uniform at ~50 µm, and the pores are very large. Most polymer-based resins have a shell on the surface that large ...


schedl_b_and_w.jpg?width=100&auto=webp&quality=80&disable=upscale)