November-December 2020
As 2020 draws to a close, we can’t help looking back at this year in which the biopharmaceutical industry has received more attention than ever before — scientifically, politically, and otherwise. We editors always have been conscious of (and pretty good at) separating our work and home lives. As many of you have learned this year, that’s an important part of working from home, which we’ve been doing for over a decade. But in recent months, reading the news and scrolling through social media seem to have become an extension of our work.
We’ve often wished that the general public better understood the realities of the biopharmaceutical industry. Before 2020, the attention you did get tended to be related to high prices and questions over bioethics. Now we see how that general lack of understanding translates to impatience with vaccine and treatment development timelines as well as legitimate concerns over safety and the supply chain. The outward messaging of your major lobbying organizations — the Biotechn...
Your boss’s boss just gave you the mandate to procure equipment, components, and raw materials for development and production of a new vaccine. Your goal is to get those as soon as possible — in addition to securing a massive volume for the future.
You and your company are in the spotlight, and under significant pressure to deliver. You simply cannot fail. Yet many other players in your industry find themselves in the very same situation. Demand is exploding, and the supply chain is broken because your supplier finds itself in the same position as its suppliers. You have no time to think or analyze. You absolutely need those components and raw materials on time. You don’t want to lose face, and you are afraid of getting ripped off. You feel that you have no power.
Does that sound like the best environment in which to conduct a successful negotiation? It feels more like trying to navigate a hurricane without capsizing your boat, which is a very stressful environment to be in, even for the most seasoned pro...
Part 1 of this article focused on the first two sessions of a CASSS chemistry, manufacturing, and controls (CMC) forum entitled “Next-Generation Biotechnology Product Development, Manufacturing, and Control Strategies,” which took place on 16–17 July 2018 in Gaithersburg, MD. Those sessions focused on upstream and downstream process technologies and strategies (
1
). Part 2 highlights the final two conference sessions on process modeling, control, and analytics.
Process Modeling and Control
The third conference session was “Modeling and Control Strategies.” Advancements in analytics and modeling and their application to biotechnology products are driving a resurgence in endeavors to transform process development and control strategies. The strategic use of analytical methods, data, and models to drive rapid advancements in process understanding, adjustments to manufacturing operations in real time, and approaches to real-time release testing (RTRT) can save resources, speed up development cycles, and incr...
Before a medicinal product can be considered suitable for patients, it must go through laborious testing and cost-effectiveness analysis. In addition, all medicinal products must be authorized before they can be sold on the market and thus made available to patients (
1
). This is the case in the European Union (EU) and European Economic Area (EEA) countries as well as in the United States (US).
Every year, a number of medicines receive marketing authorization. In their wake, however, several thousand drug candidates fall by the wayside (
2
). The discovery and development journey through to approval and marketing (Figure 1) of successful candidates takes over 12 years (often much longer) and costs about US$2.6 billion (
3–5
). In fact, for every 25,000 molecules that start in drug-discovery laboratories, only 25 will be tested in humans, and only five will make it to market, with just one drug recouping its development costs successfully (
1
).
Figure 1: Drug development overview
Both the EU and US regul...
Design for manufacturing (DfM, also known as
design for manufacturability
) is a common approach in engineering industries when complex, multistep production processes are developed and installed to manufacture products. Adherence to DfM approaches has been prevalent for decades in the automotive, aerospace, and electronics industries, among others (
1–3
). Recently, a generalized manufacturability-assessment tool with strategies to weigh different aspects of manufacturing has been proposed with numerous similarities to that described herein specific to the field of bioprocess development (
4
). Although the specific details and design goals of a DfM approach vary by industry, general principles of manufacturability prevail throughout. They are best captured by Shankar and Jannson, who define
manufacturability
as “the ability to manufacture a product to obtain the desired quality and rate of production while optimizing cost” (
5
).
Explicit discussion about the application of DfM principles in the biot...
Chimeric antigen receptor (CAR) T cells first entered US clinics in 2017 (
1
), and this therapeutic modality holds tremendous potential as one of the most effective forms of personalized cancer care ever to reach patients. The revolutionary impact of CAR T-cell therapy comes from its ability to rewire our own immune defenses to kill cancer cells: It essentially modifies a patient’s naturally existing immune cells to boost their recognition and attack of cancer cells so that the person’s own immune system can fight the disease (
2
).
Although CAR T-cell therapy is incredibly promising, production of CAR T cells presents unique challenges because these cells can’t be treated as a typical drug. They are living cells with unique biology that is not completely under our control — hence the term “living drug.” Consequently, the process of manufacturing CAR T cells requires additional oversight and stringent control to ensure that the cells will be safe and effective.
Minimizing the Risk of Harm to Patients
The...
byPing Jin
Figure 1: Size-exclusion chromatography (SEC) profile shows three main regions: high–molecular-weight (HMW) species, the product main peak, and low–molecular-weight (LMW) species. The blue chromatogram is a typical product profile; the red one is the atypical profile demonstrating high LMW with a lower product peak and no apparent change to the HMW peak.
Part 1 of this two-part report describes an investigation into the potential cause(s) and ways to control a product quality attribute (PQA) of a protein expressed in perfusion cell culture (
1
). The presence of low–molecular-weight (LMW) species following size-exclusion high-performance liquid chromatography (SEC-HPLC) is a protein quality attribute that can indicate an increase in truncated forms of the expressed protein and/or other LMW moieties. The expressed protein in this study is a heavily glycosylated recombinant glycoprotein (rGP) comprising two subunits: a 210-kDa heavy chain (HC) and an 80-kDa light chain (LC).
During a routine cell culture ca...
Several statistical techniques can be used to assist in monitoring biopharmaceutical product quality attributes as part of continued process verification (CPV) activities. These include run charts, control charts, and capability analyses. Below, I provide an overview and recommendations on statistical strategies when developing a CPV program, considering the expected behavior of manufacturing results in the biopharmaceutical industry.
Presence of Autocorrelated Data
In a previous study, I highlighted the tendency for data to be positively autocorrelated (values are closely related to each other in sequential order) in biopharmaceutical manufacturing processes (
1
). Ideally, each attribute identified in a CPV plan would be assessed to determine the potential causes of serially dependent data. A statistical approach then would be implemented to accommodate that behavior (e.g., control charting the residuals from a fitted time series model) (
2
). In practical terms, however, that is not feasible given the ...
As far back as 10 years ago, antibody–drug conjugates (ADCs) were being talked about as the next big breakthrough in pharmaceuticals due to their highly targeted approach to therapies, especially in areas such as oncology. When Adcetris (brentuximab vedotin) became the second ADC approved by the US Food and Drug Administration (FDA) in 2011, it was predicted to be a watershed moment when the floodgates would open for more approvals and novel ADC approaches. Despite that possibility, the approval of nine ADC therapeutics since 2011 has occurred only within the last two years. Alongside the increasing number of approvals, we have also seen an expansion in therapeutic uses for ADCs outside of oncology (including for inflammation and rheumatoid arthritis), new bioconjugate combinations, and a larger number of payloads and linker alternatives.
If we dig a little deeper, it is clear that the recent wave of approvals is no rarity, and our excitement for the future of ADC therapeutics is now justified. There are ...
Almac Sciences (a member of the Almac Group) recently expanded its suite of analytical solutions to include biologics testing. This follows a 2019 expansion of the company’s facility in Athlone, Ireland, where it provides a comprehensive range of flexible pharmaceutical testing services to support drug development programs adhering to industry regulations and good manufacturing practice (GMP) standards.
“Biologics have gained huge traction in the past decade and are poised for stronger growth in the coming years with potential to significantly impact patient lives,” says John Robson, Almac’s vice president of analytical operations. “The launch of this service offering further demonstrates Almac’s commitment to offering a best-in-class service to our global client base and advancing human health.”
I spoke with Pavan Kumar Kunala (biopharmaceutical laboratory manager) about the expansion and some analytical trends in the industry.
Service Expansion
Tell us about the new services Almac is offering at its Iri...
Process monitoring in laboratory and production environments enables continuous control and optimization of critical process parameters. Hence, the early detection of errors is an effective means of increasing process efficiency and reproducibility, improving quality and safety parameters, and reducing long-term costs. Highly precise and flexible noncontact clamp-on flow and bubble sensors are useful instruments to fulfill these goals. They can be applied effectively to buffer and media preparation, chromatography and filtration systems, bioreactors and fermentors, feed and transfer lines, and fill–finish applications.
Clamp-On Ultrasonic Flow Meter
An Essential Tool to Measure Flow Rates Accurately Without Liquid Contact:
One main advantage to applying ultrasonic technology for flow measurement is the ability to measure constant liquid flows independently of their charge, density, or viscosity (
1
). Ultrasonic liquid-flow measurement is especially useful for noninvasive applications. Noncontact flow me...
Yourway provides integrated supply-chain solutions for the global pharmaceutical and biotechnology industries. We exceed traditional offerings to provide customized support that includes warehousing and packaging, project management, planning and optimization guidance, comparator sourcing, ancillary-supply sourcing, forecasting, and returns/reconciliation management. Our single–specialized-provider approach offers clients a high level of convenience and efficiency that translates into quality, speed, and operational improvements.
A Growing Global Footprint
As we introduce new added-value solutions, expand our global footprint, and continue investing in infrastructure, technology, training, and innovation, our goal is to solidify our position as the biopharmaceutical industry’s provider of choice. We currently operate in 21 good manufacturing practice (GMP) depots strategically positioned around the world. That network gives us access to many countries through a wide range of transportation methods.
Our de...
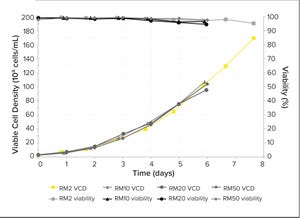
Figure 1: Viable cell density (VCD) and viability of Chinese hamster ovary (CHO) cells cultured in RM perfusion bioreactors with working volumes from 2 L to 50 L
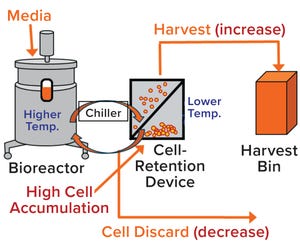
The high costs of and limits on global accessibility of biologics such as monoclonal antibodies (MAbs) are focusing the biopharmaceutical industry’s attention on strategies for rapid, economical development of such therapies. Process intensification is one approach to help shorten manufacturing timelines and reduce cost of goods (CoG) (
1, 2
). Today, process intensification in upstream cell culture enables biologics manufacturing in facilities with smaller footprints and lower scale-up volumes than was possible before. Intensified processing of Chinese hamster ovary (CHO) clones that produce MAbs is being developed in the seed train of upstream cell cultures (
3
) to support generation of high–cell-density (HCD) cell banks and processes to reduce plant size, capital investment, and overhead costs while increasing productivity.
In traditional pra...
Reliable access to high-quality starting material is a primary challenge in cell therapy manufacturing. Freshly isolated leukopak starting materials depend on donor availability and are vulnerable to cell losses from scheduling changes and other unforeseen circumstances. Flexibility often is required for cell therapy manufacturing. Therefore, relying solely on freshly isolated starting material is impractical, particularly when cell therapy logistics involve global shipping and distribution.
Donor sourcing is among the most critical factors shaping cell and gene therapy (CGT) supply chains. In the best of times, sourcing donor material for the rapidly expanding cell therapy industry is a challenge. The sheer number of CGTs in development has put pressure on starting-material suppliers, which are struggling to keep up with demand. Cryopreserved leukopaks could be an important stopgap that significantly alleviates current sourcing concerns.
Many CGT developers and research facilities already consider cryopr...
Sponsored Content
Endotoxin — also known as lipopolysaccharide (LPS) — is a large molecule made up of a lipid and a polysaccharide portion found in the outer membrane of each Gram-negative bacterial cell. Examination of endotoxins is necessary for microbial and animal cell-culture operations that produce active pharmaceutical ingredients (APIs) and vaccines. If a cell culture is contaminated with endotoxins, it could affect cell growth and function (thus potentially affecting product quality). Biopharmaceutical companies need to understand the endotoxin levels within their operations to gain more understanding of working cells.
Important for Patients:
Endotoxin is a potent pyrogen that can activate immune cells even at picomolar concentrations. The resulting release of proinflammatory cytokines into a person’s bloodstream triggers a variety of downstream immune responses, including recruitment of leukocytes and activation of the complement system. Normally, that immune response clears infections without significant collat...