September 2015
KEMWELL BIOPHARMA (WWW.KEMWELLBIOPHARMA.COM)
Both small and large biopharmaceutical companies are increasingly pursuing the outsourcing of manufacturing and testing throughout the product lifecycle. The growing use of contract manufacturing organizations (CMOs) and contract testing organizations (CTOs) has led to increasing complexity within the biopharmaceutical industry as more third-party sites are leveraged to support global markets.
To address those issues, a CASSS Chemistry, Manufacturing, and Controls (CMC) Strategy Forum was held in Washington, DC, 27–28 July 2014. The title was “Effective
Management of Contract Organizations: Sponsors, Contract Organizations, Health Authorities and Patients — Keeping the Product Pipeline Moving, Compliant, and
Available.” The CMC Strategy Forum is a series of meetings that focus on emerging and relevant CMC issues throughout a product’s life cycle. The forums foster collaborative sharing of information among industry participants and regulatory agencies. Their go...
We have had a busy summer, first adding to our annual Yearbook issue a supplement summarizing the well- attended presentations from our BioProcess Theaters at the Interphex and BIO events. And this month you are receiving our regular issue and annual guide to the upcoming BPI Conference and Exposition (25–29 October in Boston, MA) along with a sponsored supplement introducing cell-therapy initiatives at Pall Life Sciences and the third part of our special- report series featuring CMC Strategy Forum consensus papers.
Our Vendor Voice section is now called Supplier Side. We first considered this change when one long-time industry contact commented that “vendors sell hot dogs.” This new name also reflects the increased attention these days to strategic supplier–user relationships.
I am delighted this month to bring you an article from the Golden LEAF Biomanufacturing Training and Education Center at North Carolina State University’s College of Engineering. It describes an extensive program for training FDA i...
SARTORIUS AG (WWW.SARTORIUS.COM)
Sartorius Opens New Applications Center
In June 2015, Sartorius opened an application center at its North American headquarters in Bohemia, NY. Among the guests were representatives from local government, customers and business partners, and industry media including BPI’s publisher, Brian Caine. The New York Sartorius Application Center provides customers with access to multiple single-use systems for upstream and downstream processing. Demonstration laboratories present equipment by application and to show how individual systems work together for complete process solutions.
This 40,000-ft
2
facility was designed to cultivate innovation and enhance customer experience in four areas:
Mary Lavin, president of the Sartorius companies in North America, acknowledged the strategic importance of opening this center: “With this new resource, we can offer customers and employees the opportunity to see and use many of our innovative products. Our US-based R&D team is close to an im...
Photo 1: A participant samples a 300-L bioreactor in BTEC’s large-scale area.
Training and continuing education play a vital role in carrying out the US Food and Drug Administration’s mission to protect and promote the public health — not only for consumers, health professionals, and industry, but also for the agency’s own personnel. Since 2008, the Golden LEAF Biomanufacturing Training and Education Center (BTEC) at North Carolina State University has filled a niche in the agency’s internal training program and provided a series of courses to more than 100 FDA investigators. The agency’s Office of Regulatory Affairs (ORA), through its Division of Human Resources Development (DHRD), trains field and headquarters investigators and analysts to conduct inspections of biopharmaceutical and pharmaceutical manufacturing processes and practices for compliance with good manufacturing practice (GMP) and good laboratory practice (GLP) regulations.
The FDA’s ongoing need to train its field and headquarters operation...
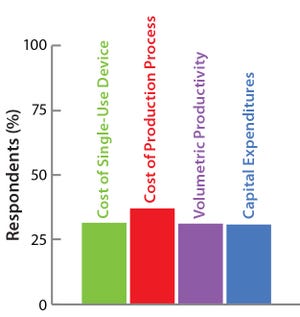
The Open Design Initiative
The BoB team has its own views on
addressing the four challenges that
must be met in designing a future
standard solution for adherent cell
culture. Market opinion is most
important, however. So the team has
conducted a series of surveys to provide
insights on design features that would
best suit market needs. A public survey
conducted in September and October of
2014 asked about the relevance of
various cost parameters to overcome
Challenge #4. Results are shown here.
The Bolt-on Bioreactor (BoB) project is an independent initiative developing and commercializing a bioreactor for efficient, automated culture of adherent cells for biopharmaceutical applications (
1
). After conducting thorough research on available culture systems for adherent cells, the BoB team believes that a successful alternative to existing devices must solve four major challenges: volumetric productivity (
2
), process automation (
3
), containment and sterility (
4
), and process economics. This month co...
www.istockphoto.com
The CMC Strategy Forums provide a venue for biopharmaceutical product discussion. They focus on relevant chemistry, manufacturing, and controls (CMC) issues throughout the life cycle of a therapeutic and thereby foster collaborative technical and regulatory interaction. Forum chairs share information with regulatory agencies to help them merge good scientific and regulatory practices. Outcomes of the forum meetings are published in
BioProcess International
and on the CASSS website (www.casss.org). This process is meant to help ensure that biopharmaceutical products manufactured with advancing technologies in a regulated environment will continue to be safe and efficacious.
This special report series highlights five general subject areas that have been covered in the first 10 years of the CMC Strategy Forum series: quality by design (QbD) and risk management; manufacturing strategies; analysis and characterization; assays, biosimilars, and comparability; and process- and product-relat...
Cosponsored by CASSS (an international separation science society) and the US Food and Drug Administration (FDA), the January 2010 CMC Strategy Forum explored antibody–drug conjugates (ADCs), which are monoclonal antibodies (MAbs) coupled to cytotoxic agents. The ADC platform of products is being used more and more for clinical evaluation in oncology. More than a dozen companies are developing several types, including products conjugated with calicheamicin, auristatins, and maytansinoids. Such products use the specificity of a MAb to deliver a cytotoxic drug to tumor cells. Depending on the chemistry of linkage, sites of attachment, and synthetic route for the small-molecule component, various chemistry, manufacturing, and control (CMC) issues arise during development and regulatory review of ADCs. Determining the appropriate assays for characterization and quality assurance (QA) is important, as is identifying potential critical quality attributes (CQAs) for the conjugate, antibody, cytotoxic agent, and ...
A CMC Strategy Forum held in Washington, DC, on Sunday 28 January 2007 focused on two topics related to protein structure and function (1). First, analytical techniques used in the glycan analysis characterization included recent advances and correlations among the various tools. And second, current understanding of glycosylation’s functional relevance to therapeutic proteins was discussed in the context of its effects on biological activity, pharmacokinetics, and Fc effector functions (for monoclonal antibodies, MAbs). Progress has been made in the field of glycobiology since this forum took place, and those updates can be found in articles referenced herein.
Two Sessions
The morning session’s goal was to develop a general understanding of the variety of analytical tools available for carbohydrate analysis: their limitations, strengths, and suitability for use in routine lot-release monitoring. The plenary session was chaired by Rohin Mahtre (Biogen Idec) and Blair Fraser (FDA CDER), with three speakers:
On 6 January 2003, 129 attendees participated in the second Well-Characterized Biotechnology Product (WCBP) Chemistry and Manufacturing Controls (CMC) strategy forum, titled “Analysis and Structure Characterization of Monoclonal Antibodies (MAbs),” held in San Francisco to discuss lot release and characterization test issues specific to MAbs (
1
).
The objective of the meeting was twofold: to identify a “core” set of assays most useful for lot-release testing of MAbs and to define a mechanism for selecting appropriate potency tests. Two separate workshops were held as a part of the strategy forum discussing these topics in detail. The purpose of this article is to describe the discussion that occurred and to define the “core” set of assays that are most frequently used for MAb characterization and lot-release testing.
Two Sessions
The morning session featured talks by three experts using a case study format to provide an industry perspective and approach to the test methods used for characterization and ...
The January 2005 CMC Strategy Forum was devoted to a discussion of live virus vaccines and viral vectors used for gene therapy. The purpose of the meeting was to determine whether consensus positions could be reached among the delegates regarding lot release, stability, characterization, and comparability testing. Part 1 of this two-part report on that meeting describes factors influencing the choices of lot-release assays for vaccines and gene-therapy products (
1
). Part 2 presents potency testing, characterization, and comparability studies, including case studies and discussion (
2
).
Two Sessions
The morning session featured plenary presentations by Keith Peden (CBER, Office of Vaccines Research and Review) and Denise Gavin (CBER, Office of Cellular, Tissues, and Gene Therapies). Peden presented an overview of factors that influence the choice of lot release assays for vaccines. Gavin described how gene therapy products are delivered through a vector with the intent of directly expressing a gene in ...
www.photos.com
Generating a stable environment for a biopharmaceutical drug substance is a critical step for ensuring a long drug-product shelf life (
1–6
). This process begins early in development with preformulation screening. Some of the most critical parameters to maintaining potency and activity are protein conformation (tertiary or three-dimensional (3-D) structure), folding (secondary structure), and proper subunit association (quaternary structure). Collectively, those are known as higher-order structure (HOS) and can be highly influenced by the formulation environment of a protein drug product.
But analytical monitoring of protein conformation is frequently omitted from preformulation because of time constraints, the complexity of most common techniques, and the inherent lack of sensitivity of some biophysical methods to subtle structural changes. However, combining traditional biophysical techniques with more sensitive orthogonal approaches can provide a comprehensive view of HOS in biopharmace...
Cells have become essential in modern medicine as therapies, vehicles for producing high‑value therapeutics, and tools for high‑throughput screening of pharmaceutical compounds. In the latter area, more than 50% of drug discovery screens use cell‑based assays, predominantly targeting receptors and ion channels using fluorescence‑based measurements, in either or both high‑ throughput and high‑content formats (
1
). Alongside a cell therapy market estimated to be worth some $5.0 billion by 2015 (
2
), the larger cell‑ based screening market is estimated to be worth $14.8.0 billion by 2018 (
3
).
Half of all screening groups express interest in using primary cells, stem cells, and early progenitor cells in their screens rather than cell lines (
4
). Such cells have close physiological similarities to cells found in actual tissues. That presents a range of challenges because such sources are known to be highly susceptible to batch‑to‑batch variability. A well‑ documented example is the use of human primary li...
The goal of formulation development for therapeutic proteins is to find conditions under which a protein remains stable during storage, transport, and delivery to patients. Both chemical and physical stability must be considered. Chemical stability is related to the rates of chemical modification to a protein molecule such as deamidation of aspargine residues and oxidation of methionine residues (
1
,
2
). Particularly important to control if they affect biological function, those modifications could also lead to changes in conformation or half-life of an antibody (
3
–
6
).
Physical stability refers to the conformational and colloidal stability of a protein. Conformational stability is its ability to maintain native secondary, tertiary, and quaternary structures. Proteins with nonnative conformation are more prone to aggregation, which can increase immunogenicity (
7
–
9
). Colloidal stability describes the tendency of native molecules to self-associate in solution, which when strong enough leads to hig...
Photo 1:
Pall Allegro STR bioreactor
The benefits of single-use technologies in both upstream and downstream operations are now widely acknowledged by the biopharmaceutical industry, and have led to radical changes in the design and operation of many bioprocesses. Those changes typically provide more robust processes and increased production flexibility. For mammalian cell culture, cleanable multiuse glass or stainless steel stirred-tank reactors (STRs) have been used successfully for growth of suspension-adapted cell lines in both small- and large-scale systems. However, achieving the same or better performance from a single-use bioreactor presents a significant challenge to product designers and developers (
1
).
Here we discuss the major design features of a 200-L single-use stirred-tank bioreactor (Pall Allegro STR 200) and present results of studies on its fluid dynamics through measurement of physical parameters that are critical to mammalian cell culture. Our studies confirmed the following:
Our r...
I often ask people: “Do you think medical practice is better, worse, or the same compared with what it was when I graduated from medical school nearly 50 years ago?” The most common answer is “I guess it’s somewhat better,” although some say “Worse!” I explain that the unequivocal right answer is “hugely better!” For example, our average lifespan is now a decade longer, and we suffer far less from incapacitating diseases such as arthritis. Then I ask, “Why are things so much improved? Is it because of doctors, medical school research, or government?” The usual reply is “I guess it’s all those things.” And I ask, “Is anything missing from that list?” Silence ensues. “What about the medical products industry?” I reply. Responses range from “I guess it produces some new technology” to “Don’t even get me started about pharma!”
I imagine BPI readers believe that they work for a fundamentally honorable industry, that they create value for society, and that they might find it strange and sad that public opinion ...
In June of this year, Leah Rosin, BPI’s marketing and digital content strategist, spoke with Rasmus Hother Le Fevre, managing director and corporate vice president, Novo Nordisk Pharmatech A/S (formerly FeF Chemicals A/S), about the company’s rebranding initiative. She began by asking him about the company’s history and reason for the name change to Novo Nordisk Pharmatech A/S.
Le Fevre:
Novo Nordisk Pharmatech was established in 1949 as FeF Chemicals, acquired by Novo Nordisk in 1986, and has been part of the company since then. On 1 September 2015, the company’s name was changed to Novo Nordisk Pharmatech. For the past 10 years, we have moved toward becoming a full-fledged pharmaceutical company and changed our product offerings, a transition that is finally completed. It was our wish to find a name that mirrors our focus and future aspirations. Our new name reflects the connection to our parent company, Novo Nordisk, and at the same time it reflects our business focus on the pharmaceutical and biophar...