Comprehensive Hands-On Training for Biopharmaceutical Manufacturing: BTEC’s Program to Deliver Training to FDA InvestigatorsComprehensive Hands-On Training for Biopharmaceutical Manufacturing: BTEC’s Program to Deliver Training to FDA Investigators

Photo 1: A participant samples a 300-L bioreactor in BTEC’s large-scale area.
Training and continuing education play a vital role in carrying out the US Food and Drug Administration’s mission to protect and promote the public health — not only for consumers, health professionals, and industry, but also for the agency’s own personnel. Since 2008, the Golden LEAF Biomanufacturing Training and Education Center (BTEC) at North Carolina State University has filled a niche in the agency’s internal training program and provided a series of courses to more than 100 FDA investigators. The agency’s Office of Regulatory Affairs (ORA), through its Division of Human Resources Development (DHRD), trains field and headquarters investigators and analysts to conduct inspections of biopharmaceutical and pharmaceutical manufacturing processes and practices for compliance with good manufacturing practice (GMP) and good laboratory practice (GLP) regulations.
The FDA’s ongoing need to train its field and headquarters operational personnel in biotechnology and pharmaceutical topics, coupled with the increasing number of biopharmaceutical products on the market and the rapid introduction of new processing technologies, led the agency to contract with BTEC to develop and deliver a customized training program. BTEC’s training courses, which provide investigators with a greater understanding of the production of biological products, are offered as part of the DHRD’s continuing education program for ORA personnel. With input from the FDA, BTEC has developed a series of four courses that cover biopharmaceutical processing from vial to vial (start to finish): Upstream Bioprocessing, Downstream Bioprocessing, Quality Control (QC)/ Analytical, and Aseptic Processing.
As a whole, the BTEC training program encompasses biopharmaceutical products, their classification, fundamental concepts underlying operations used to manufacture these products, critical process parameters, procedures, equipment design, facility design, process validation, analytical testing, and method validation.
BTEC’s mission is to meet industry’s need for professionals with highly specialized skills by providing high quality, hands-on education and training for university students and incumbent workers in all aspects of the manufacturing of biological products. The BTEC facility, which is located on NC State University’s Centennial Campus in Raleigh, has a gross 82,500 ft2 of laboratory, classroom and administrative space and is the largest such training center in the United States. Its laboratories are equipped with over $15 million of industry-standard bench- (2 L), intermediate- (30 L), and pilot- (300 L) scale bioprocessing equipment. Important features of the center include its simulated CGMP 300-L scale pilot-plant facility capable of producing biopharmaceutical products and its advanced analytical laboratory that enables complete characterization of biopharmaceuticals. (Use of the term simulated CGMP indicates that only those CGMP requirements deemed critical to BTEC’s training and education mission have been implemented in the pilot-scale facility. Consequently, products for human use cannot be manufactured in the area.) In addition, BTEC’s teaching faculty and operational staff have considerable industry and/or relevant laboratory expertise in the manufacture of biological products. This expertise informs all teaching activities.
Blended Design Delivers Online and On-Site Learning
The FDA’s ORA field employees are dispersed across the country (and in Puerto Rico) and have assignments that often require travel — factors taken into account in the design of the BTEC training program. Each of the four training courses uses a hybrid or blended design that combines online and on-site instruction, minimizing time away from work while enabling participants to complete much of the course material when and where they choose. Each course lasts five weeks and provides 36–40 hours of instruction: 12 hours delivered online and 24 hours (for the Downstream Bioprocessing and QC/Analytical courses) or 28 hours (for the Upstream Bioprocessing and Aseptic Processing courses) of face-to- face instruction that takes place at BTEC.
Each course begins with an online segment, which allows for self-paced study over four weeks. Participants must complete the online materials, including associated quizzes, before the on-site portion of the course. In addition, participants are required to attend a live two-hour, instructor-led webinar during the online portion of the course. The online course component focuses on the conceptual and theoretical knowledge associated with key topics and ensures that participants are prepared for the hands-on laboratory activities that take place on site. At least 75% of the time at BTEC is devoted to hands-on laboratory exercises; the remainder is devoted to lecture, discussion, and a variety of small-group problem- solving activities.
Even though each course stands alone, participants enroll as part of a cohort and take all four courses that make up the program during a one-year period. The number of participants in each cohort is limited to 15 to ensure that each student has a hands-on experience in the laboratory.
The online portion of each course is divided into three modules. Table 1 summarizes those modules.

Table 1: Online modules and corresponding laboratory activities for each of the four courses in the FDA training program
Course Topics
The Upstream Bioprocessing course, the first course of the program for each cohort, begins with an overview of biopharmaceuticals, including types of products produced. As shown in Table 1, the first online module covers basic concepts for the most common biological production platforms: microbial and animal hosts. The second module covers equipment (both stainless steel and disposable bioreactors), modes of operation, and critical process parameters, including temperature and dissolved oxygen, required to ensure reproducible growth and product expression. The third module focuses on equipment, operations, and facilities used to support the use of bioreactors for both microbial and animal cell growth. On-site laboratory activities that support these topics are also included in Table 1 and range from counting cells at bench scale to working with a 300-L bioreactor. Photo 1 shows a participant sampling a 300-L bioreactor during one of those activities. The on-site portion of the course wraps up with an investigation into an upstream process failure (e.g., low cell density during a microbial fermentation) in which participants must identify root cause.
The Downstream Bioprocessing course focuses on unit operations commonly used for recovering, purifying, and formulating biopharmaceuticals — in particular, centrifugation, homogenization, chromatography, and ultrafiltration. The modules (Table 1) are aligned with the stages of downstream processing: module 1 covers recovery operations (centrifugation and homogenization), and module 2 covers purification (chromatography) and formulation (ultrafiltration) operations. The third module covers topics that complement the execution of processing steps, such as equipment cleaning/sanitization and step yield calculation. In addition, the third module provides a comprehensive summary of single-use options for downstream processing.

Photo 2: A student from observes the purification of green fluorescent protein from clarified lysate using anion-exchange chromatography as part of the Downstream Bioprocessing course.
For each unit operation, online material covers the fundamental concepts that underlie unit operations, critical process parameters, and design of equipment. Laboratory activities (Table 1) are related to online topics and provide hands-on experience with each of the operations included in the online material. Photo 2 shows a participant observing the anion-exchange capture chromatography step for BTEC’s Escherichia coli–based, pilot-scale green fluorescent protein (GFP) process. GFP is used as a model biopharmaceutical in the downstream laboratory activities.

Photo 3: A student loads a sample into a microchip for analysis by electrophoresis in the QC/Analytical course.
The QC/Analytical course covers key aspects of measurements used for both product intermediates and final drug products. The first module of the online material is focused on assay attributes such as precision, accuracy, specificity, linearity, and limit of detection/quantitation and basic statistical concepts relevant to analyzing analytical results. The second and third online modules deal primarily with actual assays: chemistry assays (UV/vis spectrometry, high-performance liquid chromatography (HPLC), various types of electrophoresis, mass spectrometry, amino acid analysis, total protein assays, enzyme-linked immunosorbent assay (ELISA), and glycosylation analysis); bioassays (animal model assays, various cell- line-derived bioassays); and microbiological assays (bioburden, sterility, endotoxin, mycoplasma, and virus testing). The third module also includes introductory information on biopharmaceutical formulation and stability, as well as a brief discussion of emerging analytical methods such as electrochemiluminescence-based assays and rapid microbial detection techniques. In laboratory, participants execute many of the assays covered in the online material (Table 1). Photo 3 shows a participant loading a sample into a microchip for analysis by electrophoresis.

Photo 4: Students learn about the semiautomated filling machines in the Aseptic Processing course.
The Aseptic Processing course introduces key topics associated with the filling of biopharmaceutical drug products. As in the other courses, online content is divided into three modules. The first module begins by discussing two methods for producing sterile final drug product: terminal sterilization and aseptic processing. That module also covers facility design, environmental monitoring methods and equipment, sanitants and disinfectants used in rooms, and effective gowning practices. The second module covers aseptic technique, components used in parenteral manufacturing (and their sterilization), and formulation. The third module focuses on the actual aseptic filling process, including media-fill process simulation, lyophilization, and in-process checks. Laboratory activities associated with these topics are summarized in Table 1. Photo 4 shows students working with the semi-automated fill machine used as part of the course.
Participant Profile and Selection
According to the FDA, this series of courses is intended for individuals who will perform inspections of biomanufacturing facilities and includes personnel with expertise and prior training in pharmaceutical regulation. The target audience for this training program consists of experienced consumer safety officers (CSOs) who are currently conducting sterile drug and/or biotech inspections (domestic and international). Prospective applicants, who must have supervisory concurrence, submit registration forms to their area training coordinators, who then prioritize the applications received based on criteria specified by the FDA’s Division of Human Resources Development (DHRD).
To date, a total of 103 participants have participated in the program, and an additional 15 are in progress. Among the past five cohorts (72 participants), 48.6% had over seven years of experience conducting inspections, 29.2% had four to seven years of experience, and 22.2% had less than four years’ experience, Of this group, 62.5% had completed at least some graduate education, and a number hold master’s or doctoral degrees. Their academic degrees are in biology (50%), chemistry (11.1%), pharmacy (9.7%), a variety of other sciences (20.8%), and engineering and other majors (8.2%).
Assessment of Learning and Program Evaluation
Each online module (Table 1) has its own quiz. Those quizzes, along with the self tests built into the online material, provide participants with feedback to enhance their understanding of the material. Trainees must score 80% or higher on each quiz to complete a module. Trainees who do not receive a passing grade must complete an alternative version of the quiz, which contains different questions related to the same topics. If a trainee fails the alternative quiz, the instructor intervenes and provides remediation as required. Participants must complete the online modules before coming to BTEC for on-site instruction. The two-hour webinar that the instructor leads during the online segment of the course provides another check of student understanding.
On-site assessment varies from course to course but typically includes monitoring of performance on laboratory activities, examination and discussion of data obtained during laboratory exercises, questioning (both questions posed by the instructor and question-and-answer sessions), and general discussion. Because of the hands-on, experimental nature and rapid pace of the on-site activities, participants are usually very engaged in the learning process.
Participants who successfully complete the course earn continuing education units (CEUs) ranging from 3.6 to 4, depending on the duration of the course.
BTEC also obtains participant information and seeks feedback from trainees on other aspects of program effectiveness through a series of surveys.
A background survey, completed before the Upstream Bioprocessing course (the first course in the series for a given cohort), provides instructors for all courses with a measure of their upcoming audience’s knowledge and expectations.
An end-of-course evaluation survey is made available on the last day of each course.
A follow-up survey is conducted online six months after trainees complete a course.
At the conclusion of the on-site training, trainees are asked to complete a web-based evaluation of the course. The evaluation form requests trainees’ opinions of the course content and organization, relevance of laboratory and other on-site activities, instructor preparedness and efficacy, and other factors reflective of course quality and usefulness. Among the numerous questions included, we ask students to comment on whether they have a greater awareness of and appreciation for possible failures in upstream processing, downstream process, QC/analytical, and aseptic processing. Since the program began, over 95% of students have indicated that they have a greater appreciation. In addition, when students are asked to rate whether each course provided the information and skills needed to effectively carry out their responsibilities, over 95% agree or strongly agree.
Each course evaluation also includes items that are intended to measure anticipated impact of that particular course. Trainees are asked to indicate on Likert scales the frequency with which they exhibited in the past certain behaviors related to course content and intend to exhibit those behaviors in the future. For example, trainees who complete the Upstream Bioprocessing course are asked to indicate how often, before the course, they examined data describing the kinetics of cell growth in individual batches and how often they will do so in the future. To gauge ongoing changes in behavior, trainees are again surveyed six months after completing the course and asked how often they actually exhibited those behaviors in the intervening period. Table 2 shows responses to these statements indicate some shift of behavior resulting from this program. In addition, we have heard anecdotal evidence from participants suggesting that the program is having an impact on what investigators are looking at during inspections.
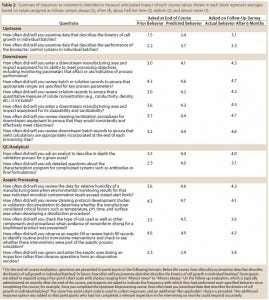
Table 2: Summary of responses to statements intended to measure anticipated impact of each course; values shown in each block represent averages based on values assigned as follows: almost always (5), often (4), about half the time (3), seldom (2), and almost never (1).
In the seven years this training program has been held, feedback from these surveys, informal discussions with participants, and internal reviews by the FDA have led to a number of improvements/changes in several areas. These include the following:
Expansion of the Upstream Bioprocessing and Aseptic Processing courses to allow for an additional half day of on-site activities
Addition of viral clearance as a topic in the online materials for the Downstream course, and addition of on-site material related to downstream process validation
Elimination of unnecessary redundancies in topics between courses, such as environmental monitoring, which had been covered in both the QC/Analytical and Aseptic Processing courses.
Lessons Learned
A number of lessons have been learned since the program’s inception:
Using standardized instructional and visual designs for the online portion of all courses in the program provides a consistent user interface, which increases usability.
Despite the fact that completion of online material by participants is required before coming to BTEC, a review of relevant fundamental concepts just before laboratory sessions results in better laboratory execution and gives students a better understanding of the overall goals and design of the laboratory activity.
Managing the online portion of each course requires significant attention just like execution of the laboratory activities. Identifying one person on the provider’s end to serve as the online course “manager” and handle the myriad of tasks (enrolling students, tracking student progress, providing information and updates to the FDA, and addressing questions not related to course material) leads to a well-organized course and efficiency in delivery.
Course Summary
BTEC has developed and delivered a comprehensive training program in biopharmaceutical manufacturing and testing for FDA investigators consisting of four courses: Upstream Bioprocessing, Downstream Bioprocessing, QC/Analytical, and Aseptic Processing. Participants enroll as part of a cohort and typically take all four courses during a one-year period. As a whole, the program covers biopharmaceutical products, fundamental concepts underlying the operations used to manufacture those products, critical process parameters, procedures, equipment design, facility design, process validation, analytical testing, and method validation. Postcourse evaluations show that overall participant satisfaction with the program is high and that participants are expanding the scope of their inspections, a key objective of the program.
The program at BTEC continues to evolve. For example, topics continue to be added to the course material, such as viral clearance and process validation. Existing course content is frequently updated, particularly for topics involving technologies that are changing rapidly such as single-use options and rapid microbiological assays.
Acknowledgements
BTEC appreciates the continuing support of the US Food and Drug Administration and FDA project officers Karen Coscarelli, Owbra McNary, Michael Chasey, and Constantine Markos. The program described is funded by contract number HHSF223201210130C from the FDA Office of Acquisition and Grants Services (OAGS), Division of Contracts and Grants Management (DCGM). The authors also wish to acknowledge the significant contributions that the following people have made to the development and delivery of this training program: Dr. John Sheppard, Dr. Nathaniel Hentz, Dr. John van Zanten, and Dr. Mike Morgan.
Disclaimer
This article reflects the views of the authors and should not be construed to represent the FDA’s view.
Corresponding author Gary L. Gilleskie, PhD ([email protected]) is director of operations and teaching associate professor, and Patty Brown (patty_ [email protected]) is a senior instructional designer at Golden LEAF Biomanufacturing Training and Education Center, College of Engineering, North Carolina State University, Campus Box 7928, Raleigh, North Carolina, United States 27695-7928.
Photos courtesy of BTEC. Copyright 2010– 2014 North Carolina State University.
You May Also Like






