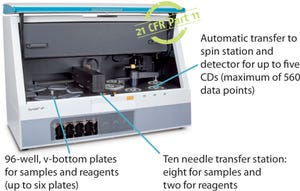
Increasing efficiency and productivity for titer and impurity bioanalytics is even more critical as a result of increasing workloads in supporting multiple programs with limited resources. Current methods such as enzyme-linked immunosorbent assay (ELISA) or high-performance liquid chromatography (HPLC) create bottlenecks in critical workflows because of throughput constraints, repeat testing, or longer assay times. The Gyrolab™ workstation is an automated immunoassay platform that can generate high-quality analytical data at high throughput using nanoliter volumes for more rapid and efficient titer and impurity immunoassays.
Gyrolab offers key advantages for applications such as protein expression, cell line development, and the detection of bioprocess impurities. It is a robust, automated platform providing high throughput, enabling data-driven decisions with minimal bench time and rapid time to results.
Gyrolab xP Workstation
Gyrolab xP workstation is an automated platform that uses proprietary microflu...
From drug and biologic development services to delivery technologies to supply solutions, we are the catalyst for your success. With over 80 years of experience, we have the deepest expertise, broadest offerings, and the most innovative technologies to help you get more molecules to market faster, enhance product performance, and provide superior, reliable manufacturing and improved results. Catalent is your strategic partner for biologic drug development success.
SMARTag™ ADC Technology for the Development of Optimized Antibody–Drug Conjugates:
Catalent has an exclusive license and comarketing agreement with Redwood Bioscience to offer customers SMARTag advanced, site-specific conjugation technology and linker–toxin combinations to develop optimized antibody–drug conjugates.
SMARTag proprietary technology can help the development of improved ADCs. It allows for precise conjugation and optimal release of the therapeutic agent, and can provide
Biomanufacturing Center of Excellence:
Our state-of-the-art f...
Environmental monitoring of clean rooms is an integral part of a pharmaceutical company’s quality control testing program. Analyzing the air, surface, and personnel within cleanrooms helps ensure the manufacturing environment meets mandated standards and that any variations can be addressed. Unfortunately, the traditional method for environmental monitoring is manual, inefficient, and time consuming. Laboratory technicians must travel, sometimes across large campuses, to collect samples in manufacturing suites. Technicians have to gown, take the samples, and travel back across the campus to the lab where samples are logged in before incubation.
After 5–7 days, the samples are removed, and each plate is manually counted. Technicians then record the counts for each plate. Paper results are keyed into a laboratory information system (LIMS). For large sites, environmental monitoring programs involve hundreds of samples being tested each day, taking time and resources that could be used for higher value activ...
Following the initial observations by Chen et al., leading manufacturers of biopharmaceuticals have determined that endotoxin (LPS) can be “masked” in biopharmaceutical formulations typically containing phosphate, polysorbate, and sugars (
1
). Being in a masked state, endotoxin is undetectable by the commonly used factor C detection methods such as the Limulus amebocyte lysate test (LAL). This phenomenon has also been denominated low endotoxin recovery (LER).
As previously shown by Reich et al. (
2
), endotoxin masking is time dependent, meaning that a defined amount of endotoxin spiked to a sample at time point zero often cannot be recovered (detected) with commonly used methods after hours or days. This is sometimes the case even within minutes of positive product controls (PPCs) initially have indicated a positive test result. Such lack of test reliability can ultimately affect patient safety.
FIGURE 1: Workflow of Hyglos sample preparation method for
unmasking endotoxin
Reich has studied the mechanis...
No longer is the biopharmaceutical industry questioning whether it should use single-use technology. The focus has transitioned to how to best implement single-use systems. Rapid adoption of single-use systems has brought added complexity; which is leading to an increasing application of lean manufacturing principles.
Standardization projects and establishing appropriate standards are current trends that drive toward reducing the complexity of bioprocessing systems, from design and validation to supply chain and manufacturing integration. Although efforts are underway to develop consensus standards for topics such as extractables and leachables and particulates, a number of drug and contract manufacturers are focusing on internal projects to standardize single-use designs across their processes and facilities.
As the market matures, more companies are likely to adopt modular system designs to build the facilities that support multidrug production. Incorporating a catalog of standardized bags, tubing manif...
Advances in cell line engineering and media design have allowed for improvements in monoclonal antibody expression, with cell culture systems exhibiting product titers >10 g/L. These high-density cell cultures pose a great challenge in the use of existing solid liquid separation techniques because of the need to remove larger amounts of biomass and the increase in submicron particles. In addition, the increased level of soluble impurities introduces new bottlenecks for chromatographic processing because of the increased levels of contaminants from cell debris generated during cell culture and harvesting.
FIGURE 1: Particle-size distribution (PSD) of a representative CHO cell culture feed stream (MAb05). Data are shown for unadjusted (pH 7.0), acid treated (pH 4.8), and pDADMAC (0.0375%) treated cell culture feed streams.
The use of flocculation in harvest is a way to overcome these challenges. Controlled flocculation of cell culture suspensions can be used to enhance clarification throughput and downstrea...
As product titers continue to increase in upstream cell culture processes, so too the demand for enhanced capacity in downstream purification steps. MabSelect SuRe LX chromatography medium (resin) is specially developed for monoclonal antibody (MAb) capture from high-titer feeds. To help ensure good process economy, the medium can withstand hundreds of rigorous cleaning cycles with NaOH, with retained dynamic binding capacity (DBC) and MAb yield.
High Dynamic Binding Capacity (DBC)
FIGURE 1: MabSelect SuRe LX exhibits significantly higher DBC compared with MabSelect SuRe medium at 6 min residence time.
Large-scale purification of MAbs usually consists of two or three chromatographic steps, where protein A is the ligand of choice for the initial capture step. MabSelect SuRe LX is based on the same rigid high-flow agarose base matrix and alkali-stabilized ligand as its precursor MabSelect SuRe medium. When compared with MabSelect SuRe, however, MabSelect SuRe LX provides up to 45% higher DBC at 6 min reside...
Purification of therapeutic monoclonal antibodies (MAbs) requires advanced and selective means for separating aggregates from target monomers. Cellufine MAX GS chromatography media are optimized for higher capacity, better separation, and greater selectivity to improve downstream purification of MAbs. JNC Corporation’s new strong cation exchanger, Cellufine MAX GS, has optimized ligand density for improved dynamic binding capacity and selectivity and superior pressure flow characteristics, which are required by MAb purification platforms.
FIGURE 1: Separation of aggregates by stepwise elution.
MAb load = 75 mg/mL resin; column = 5 mm ID 5 cm L, A buffer = 10 mM Na-Acetate pH 4.5 + 50 mM NaCl; B buffer = 10 mM Na-Acetate pH 4.5 + 1 M NaCl; load and wash = 0 % B, first step = 18.5 % B, second step = 100 % B; flow = 0.17 mL/min (load), 0.50 mL/min (elution); sample = HCl treated mouse MAb1 (monomer: 92.6%, dimer = 4.5%, aggregates = 2.9%.
MAb Monomer/Aggregates Separation:
Cellufine MAX GS exhibits exceptio...
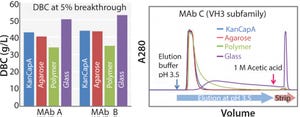
Industrial-scale production of pure monoclonal antibodies (MAbs) customers require an overall highly efficient affinity resin with a high binding capacity, high alkaline stability, good elution profile, low nonspecific binding, and large-scale column operation. Kaneka has developed a novel protein A ligand and immobilized it to highly cross-linked cellulose beads to totally satisfy all these requirements. Here, we demonstrate the performances of the Kaneka KanCapA using MAbs.
FIGURE 1: Dynamic binding capacity (for MAb A and B) and elution profile (for MAb C) of KanCapA compared with commercially available protein A resins; 5% breakthrough is determined at 6 min of residence time (left); 5 mg/mL-resin of IgG (VH3 subfamily) is loaded and elution is performed at pH 3.5. Strip solution is 1 M Acetic acid (right).
FIGURE 2: Pressure/flow rate characteristics of KanCapA packed into various column sizes; mobile phase is water. The pressures generated by packed beds are calculated by subtracting the system pres...
The LEWA EcoPrime® LPLC chromatography platform combines proprietary fluid design and the LEWA Intellidrive® technology to deliver unrivaled batch-to-batch reproducibility and accuracy. The LEWA EcoPrime® features our open architecture automation software and is built on a quality control of the industry’s most vertical supply chain. A single LEWA system has the range of up to three conventional LPLC units while providing a higher level of accuracy. This gives the user great flexibility, more floor space, and plant-friendly integration with simple software customization and updating.
We have combined state-of-the-art functionalities into one unit. It can be a buffer dilution skid or directly connected to a column to be a chromatography system. Tank requirements and labor related to buffer make-up are reduced when the unit directly connects to supplies for purified water and water for injection. This system has been optimized through software analysis of fluid motive, fine-tuned over our 60-year history to...
Strong anion-exchange (Q) chromatography is an industry standard for polishing purification steps of monoclonal antibody (MAb) production. It is a proven technology that removes DNA, viruses, endotoxins, and acidic host-cell proteins (HCPs) from process feed streams in flow-through mode. Advancements in cell culture technology, the emergence of cost-sensitive biosimilars, and the limitations of conventional purification technologies have led to an industry-wide interest in downstream single-use technologies and more flexible biomanufacturing options.
Despite their high binding capacity, traditional resin-based chromatography columns are often oversized because of throughput limitations and are not suitable for flexible biomanufacturing. Conventional membrane adsorbers, meanwhile, offer faster throughput and a certain amount of flexibility, but they cannot provide sufficient process robustness because of the low binding capacity typical of most membranes. These factors impose challenges on the design of pu...
Given the current drive to reduce the cost of manufacturing for biological therapeutics, it is incumbent upon chromatographers and process engineers to streamline the manufacturing process wherever possible. As a result of this need, the demands on downstream unit operations have increased to the point where a single processing step is expected to accomplish a multitude of purification objectives.
Introduction
Anion exchange chromatography is a widely used technique for protein capture or impurity removal. Typically, anion exchange resins with quaternary amine or DEAE ligands are used. However, these more conventional resins have the disadvantage of reduced capacity for proteins in relatively high salt concentrations associated with post-protein A purification monoclonal antibodies (mAbs) or undiluted biological feedstock. In order to use a DEAE or quaternary amine resin at these process stages, the column load material must be diluted to adjust its conductivity to approximately 5 mS/cm.
TOYOPEARL NH2 -75...
Membrane chromatography is an effective alternative to conventional beaded sorbents for the purification of large virus molecules or viral-like particles. Membranes have an open pore structure that allows fast kinetics and direct access to ion-exchange binding sites, with flow rates that are orders of magnitude faster than conventional columns. They provide much higher binding capacities than beaded sorbents for large molecules limited by mass transfer. Pall Mustang ion-exchange membranes are scalable membrane chromatography devices used in various therapeutic, vaccine, and gene therapy products purification schemes. These ready-to-use devices (reusable or single-use) allow users to significantly reduce hardware investments and validation costs typically associated with column chromatography.
A study performed on live influenza (H1N1) showed how structured design of experiments (DoE) and high-throughput screening (HTS) experiments performed on AcroPrep Advance 96-well filter plates with Mustang Q membrane...
This application note describes a case study of the correct sizing for a filter involved in an automated final bulk-fill system for a biopharmaceutical product. It demonstrates how customer batch-processing requirements can be attained with a robust experimental design.
Scale-Up Methodology
Figure 1 shows a process for successful scale-up. Once the desired process has been defined, filter performance can be determined by either constant flow or constant pressure testing on disc formats.
FIGURE 1: A process for successful scale-up
The data can be scaled-up easily to determine the required system size for the target process because capacity is directly proportional to filter area. Pilot-scale or full engineering trials can be used to confirm initial findings because experimental variability can be introduced through differing filter formats, drug product batches, or process equipment.
Sizing should be performed using a scale model of the target process. Ideally, processing time should remain constant, and t...
The increasing adoption of single-use technology in biopharmaceutical, vaccine, and cell therapy production is one indication that such technology has moved far beyond its novelty stage. Arguably, this is the preferred technology of newly developed processes. To support the continued growth of single-use systems in the biopharmaceutical industry, the ability to measure critical process parameters with single-use sensors is required. Several key single-use sensing technologies are described, including pressure, temperature, UV absorbance, and conductivity. The technology behind each is explored as well as examples of the sensors in practical applications.
Pressure Sensors:
Single-use sensors for pressure measurement have already become widely accepted and adopted. This is partly attributed to the fact that pressure is the most critical measurement related to process performance (as well as safety) for many different bioprocesses. Products such as PendoTECH single-use pressure sensors are used to measure p...
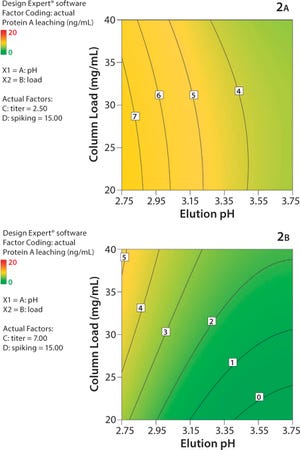
Protein A chromatography has become a widely used platform in monoclonal antibody (MAb) purification. We describe the impact of higher MAb loadings on aggregation and ligand leaching during protein A chromatography, as well as the benefits of high-capacity Protein A resins for high-titer feedstocks. Today, high MAb titers achieved through advanced upstream processing have become a challenge for downstream processing, especially with regards to the expensive protein A capturing step. TOYOPEARL AF-rProtein A HC-650F provides superior MAb capacity and beneficial MAb uptake behavior and can thereby increase capturing productivity.
MAb Adsorption:
Because dynamic capacity depends on flow and feed concentration, it was tested at various residence times (RT) and MAb titers. Figure 1 shows the breakthrough curve for TOYOPEARL AF-rProtein A HC-650F at a feed concentration of 10 g mAb/L. The resin shows complete MAb adsorption until breakthrough occurs even for short RT of 1 min. The capacities of more than 100 mg...
Biosimilars are set to dramatically improve how millions of people access expensive, complex, life-saving treatments by providing equally effective alternatives to innovator products while lessening the cost burden (>30%). Therapeutic Proteins International, LLC (TPI) is a fully integrated manufacturer of biosimilar recombinant protein products that has reinvented conventional approaches to biologic drug manufacturing.
Powerful Science Shouldn’t Be Complicated
TPI has reinvented the process used to manufacture biological drugs making it simpler by leveraging superior technology and rigorous scientific methods to significantly reduce the number of steps involved. At the core of TPI’s patented manufacturing platform are disposable bioreactors and a totally single-use system. Those proprietary systems allow for quick scale up and simultaneous development, which reduce the timeline and costs associated with bringing products to market. In addition, TPI performs analytics at each step to optimize performance a...
Sponsored Content
Major Markets
AbbVie Contract Manufacturing serves domestic and global companies seeking to outsource biologics, potent capabilities, drug products, fermentation, prefilled syringe manufacturing, and HME, as well as businesses looking to procure APIs. Outsourcing clients range from mid-size to large pharmaceutical and biotech companies.
Services Offered
Biologics:
AbbVie is a leading biopharmaceutical developer and manufacturer, with a scientific team that offers expertise in process optimization, analytical characterization, and current good manufacturing practice (CGMP) manufacturing to support full development and commercialization of biologics.
Potent Capabilities:
AbbVie Contract Manufacturing offers a full range of potent capabilities for drug product and highly potent active pharmaceutical ingredients (HPAPIs), covering development phases to commercial equipment capable of handling potent classification of 3.
Drug Products:
AbbVie Contract Manufacturing offers a full range of drug product manufa...
Aeras is a nonprofit biotech focused on the development of TB vaccines. Together with our global research and development partners, we have played an integral role in developing approximately half of all TB vaccine candidates in clinical development around the world.
TB is one of the most deadly infectious diseases in the world. It spreads through the air like the common cold. A TB patient with active disease can infect up to 15 people simply by coughing, sneezing, or talking. The emergence and spread of drug-resistant forms of TB are complicating multinational efforts to control the global epidemic.
Because TB is more complex and more difficult than ever to treat, it presents a worldwide health threat. So attacking this challenge requires developing expertise and experience in multiple biologics technology platforms. Aeras approach includes building integrated, state-of-the-art, end-to-end development and manufacturing capabilities for translational development support all under one roof.
Aeras is levera...
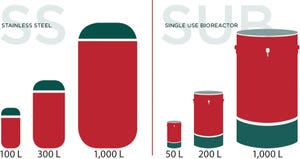
Avid Bioservices is a contract manufacturing organization (CMO) specializing in mammalian cell culture process development and current good manufacturing practice (CGMP) production of clinical- and commercial-scale monoclonal antibodies, recombinant proteins, and enzymes. Committed to your success, our team is constantly striving to build partnerships that extend well beyond the delivery of product. By combining our knowledge and experience with a flexible and efficient approach, we can meet your specific project requirements.
Established CMO with Proven Capabilities
Excellence in Process Science
FIGURE 1: Bioreactor capacity
Avid’s process sciences group is focused on the development of process and analytical methods needed to deliver active products for transfer to CGMP manufacturing. Our experience with CHO-based IgG production, as well as knowledge in the areas of recombinant protein and enzyme process development, provide the expertise you need to advance your programs to the next phase. Expertise in...
Boehringer Ingelheim BioXcellence™ is your dedicated biopharmaceutical contract manufacturer. As a leading biopharmaceutical contract manufacturer with more than 35 years of experience, it has brought 22 biopharmaceutical products to market. Boehringer Ingelheim BioXcellence™ offers tailor-made contract development and manufacturing services to the biopharmaceutical industry, providing the entire production technology chain from DNA to fill and finish under one roof at its facilities in Biberach (Germany), Vienna (Austria), Fremont (USA), and Shanghai (China). Boehringer Ingelheim BioXcellence™ can secure product supply throughout the entire product life cycle transferring customer projects at any stage, delivering to almost any scale. Boehringer Ingelheim BioXcellence™ makes outsourcing easy.
Boehringer Ingelheim BioXcellence™ Services
At our cell culture sites in Biberach (Germany) and Fremont (USA), we have a total capacity of more than 200,000 L, with flexible scales for clinical and commercial supply...
Bosch Packaging Technology has been supplying the pharmaceutical industry with final fill solutions for over 30 years. During that time, Bosch has continued to focus on quality and industry-leading customer-focused solutions that provide the best return on investment over time.
Filling Systems
Bosch is the premier supplier of final fill–finish solutions to the pharmaceutical industry.
As a single-source supplier with the industry’s widest portfolio of solutions, Bosch can supply the type and scale of equipment you require. We offer systems for bulk syringes, vials, ampules, cartridges, and prefilled syringes from laboratory scale to high-speed production. Our project management team will work with you as your system is pretested in our facility to ensure that it performs as promised before delivery.
Sterilization Systems
With the acquisition of SBM, Bosch now offers a full line of sterilizations systems.
Specially made for the rigors of the pharmaceutical industry, Bosch sterilizers are designed for max...
Expertise
Cobra Biologics is a rapidly expanding international contract manufacturing organization (CMO) of biologics and pharmaceuticals for clinical and commercial supply. Cobra has three good manufacturing practice (GMP)-approved facilities, each with expertise tailored to serving our customers across the world. We offer a broad range of integrated and stand-alone contract services, stretching from cell line and process development through to fill and finish for the supply of investigational medicinal products and commercial production.
Experience
For over 16 years, we have taken pride in being a trusted manufacturing provider, delivering what we promise and helping our customers to develop medicines for the benefit of patients. Cobra provides manufacturing technologies, platforms, and solutions to the biopharmaceutical industry covering antibodies, recombinant proteins, viruses, phage, DNA, whole-cell vaccines and therapeutics as well as biologics and small-molecule active pharmaceutical ingredient (A...
The commercial prefilled syringe line at Cook Pharmica can process up to 600 syringes per minute, 36,000 syringes per hour, or 70 million syringes per year. The automated, high-speed syringe line is fully enclosed in a barrier isolator to provide the highest level of aseptic processing available by removing the human element from the entire process. With the ability to operate in smaller batches or full-scale campaigning, the prefilled syringe line is capable of bridging clinical programs into commercial production.
The process starts in a separate tub-loading room where tubs are loaded onto a pass-
through conveyor and sent into the isolator for automatic debagging. After the tub is removed from the bag, it passes through a tunnel utilizing E-beam technology for tub decontamination. When it exits the E-beam tunnel, robotic arms remove the lid and liner to prepare the syringes for denesting and filling.
The line is capable of time-pressure or peristaltic filling, with temperature-compensating recirculatio...
The ability to successfully produce cell-based products is partially dependent on the technologies and instruments available. Volume manipulation, supernatant or culture media exchange, and cell washing of the cell product can present a significant challenge, especially when cell recovery needs to be maximized without compromising viability.
FIGURE 1: SEM images of the track-etched polycarbonate membrane used within LOVO’s proprietary spinning membrane separation device; view normal to the membrane surface (left), cross-sectional view of the membrane (middle), and close-up of the 4-μm pores (right)
LOVO is a multiuse, bench-top instrument that pumps a cell solution through a spinning membrane filtration device to allow for rapid fluid management and fast, efficient cell processing. At its core, LOVO leverages Fresenius Kabi’s proprietary spinning membrane separation device, which uses a 4-μm track-etched polycarbonate membrane (Figures 1 and 2), and allows for separation of target cells through size disc...
Fujifilm Diosynth Biotechnologies (FDB) leverages over 15 years of experience in bioprocess development and manufacturing to successfully deliver robust process designs to its clients. FDB has executed a series of innovation programs to enable rapid development of cell culture processes, including the coming launch of a new Chinese hamster ovary (CHO) expression system. This application note describes how outputs from media screening and upstream process development innovation programs were applied to provide significant productivity and process robustness improvements to an early stage client program.
Results
Toolbox Media/Feed Screening: FDB developed a toolbox of high-performance basal media and feeds by screening numerous commercial and prototype media with diverse CHO cell lines.
FIGURE 1: Cell growth trends with various basal and feed media
combinations
In this case study, 10 basal media and feed media combinations were selected for fed-batch production trials in shake flasks. Varying degrees of cel...
The Xuri cell expansion systems (Xuri W25 and W5) provide a functionally closed, single-use bioreactor that requires fewer operator intervention steps, reduces the risk of contamination, and controls the cellular environment to maximize cell yield. Data presented here highlight that at a cellular level, results from different bioreactors may appear similar, but differences can exist at the receptor expression level.
Expansion of T Cells
FIGURE 2: An example of how the pO2 saturation can vary during the
expansion of T cells; the lack of effective mixing in the STR bioreactor
reduces the capability of the stirred-tank system to maintain an
acceptable level of dissolved oxygen.
FIGURE 1: An example of how pH can vary during the expansion of T
cells; at higher cell densities, the stirred-tank bioreactors, which lack
perfusion features, are unable to maintain a suitable pH, resulting in the
operator having to exchange medium to correct the pH.
Peripheral blood mononuclear cells (PBMCs) were expanded in static ...
Sponsored Content
NNE Pharmaplan has developed a new facility concept called Bio on demand™, which enables facilities to be established in one to two years. The result is a flexible facility that is fully operational, locally compliant, and contains functioning quality systems. Bio on demand™ involves a high degree of single-use technology to ensure cost-effective production and fast establishment.
Facilities based on our Bio on demand™ concept can be delivered globally, but they are primarily intended for growth markets where time-to-market is particularly essential.
Full Package for Full Predictability
The Bio on demand™ concept includes engineering and supply of a facility as well as related quality systems, standard operating procedures, and coordination of necessary quality tests.
When executing a project, NNE Pharmaplan’s overall objective is that the customer develops new capabilities and grows existing ones. And by not only providing a facility but also a full turn-key solution, we allow our customers to focus on c...
Sponsored Content
Unlike typical pharmaceuticals, cell therapies rely heavily on the manufacturing process for product attributes. As more products move out of the clinic and toward commercialization, the manufacturers of cell therapies are realizing that commercialization is unlikely to be successful without an effective methodology for manufacturing development.
At PCT, we recognize that a successful commercial manufacturing vision includes the ability to provide consistently high product quality at a reasonable cost of goods that meets demand over the commercial life of a product. To help our clients realize this vision, PCT is proud to offer its innovative scientific and engineering expertise to plan and execute development by design (DbD) methodology.
PCT’s industry-leading DbD approach applies a series of principles to our clients’ cell therapy manufacturing processes to help guide strategy and provide structure and discipline to plan for and address commercialization risks before it is too late to efficiently addres...
Pyramid Laboratories, Inc. is a contract aseptic manufacturing and analytical services organization for sterile injectable drugs. Pyramid provides expertise in formulation and process development, aseptic filling for vials and syringes, and lyophilization applications. Pyramid has established a reputation of exceptional performance, integrity,
and quality.
Technical Services
Pyramid offers a wide array of services for all phases of drug development and production including
Facilities
Pyramid Laboratories, Inc. is located in southern California in the United States. Our facilities are housed in three buildings covering more than 70,000 ft
2
. The combination of Pyramid’s manufacturing facilities and state-of the-art laboratory allow the company to offer the pharmaceutical and biotech industry both analytical and manufacturing support capabilities. An intensive in-house quality assurance and quality control program is maintained to ensure that clients receive high-quality products. Pyramid expanded its por...