- Sponsored Content
High-Capacity, Single-Use, Strong Anion-Exchange (Q) Chromatography Tools: Speed and Process Flexibility for MAb PurificationHigh-Capacity, Single-Use, Strong Anion-Exchange (Q) Chromatography Tools: Speed and Process Flexibility for MAb Purification
August 15, 2014
 Strong anion-exchange (Q) chromatography is an industry standard for polishing purification steps of monoclonal antibody (MAb) production. It is a proven technology that removes DNA, viruses, endotoxins, and acidic host-cell proteins (HCPs) from process feed streams in flow-through mode. Advancements in cell culture technology, the emergence of cost-sensitive biosimilars, and the limitations of conventional purification technologies have led to an industry-wide interest in downstream single-use technologies and more flexible biomanufacturing options.
Strong anion-exchange (Q) chromatography is an industry standard for polishing purification steps of monoclonal antibody (MAb) production. It is a proven technology that removes DNA, viruses, endotoxins, and acidic host-cell proteins (HCPs) from process feed streams in flow-through mode. Advancements in cell culture technology, the emergence of cost-sensitive biosimilars, and the limitations of conventional purification technologies have led to an industry-wide interest in downstream single-use technologies and more flexible biomanufacturing options.
Despite their high binding capacity, traditional resin-based chromatography columns are often oversized because of throughput limitations and are not suitable for flexible biomanufacturing. Conventional membrane adsorbers, meanwhile, offer faster throughput and a certain amount of flexibility, but they cannot provide sufficient process robustness because of the low binding capacity typical of most membranes. These factors impose challenges on the design of purification schemes for manufacturing biotherapeutics.
NatriFlo HD-Q membrane adsorbers overcome those limitations by combining high binding capacity and high flow rates to deliver best-in-class performance in single-use plug-and-flow format. This article compares the performance of NatriFlo HD-Q adsorbers with currently available column-based resins, anion-exchange (AEX) membranes, and salt-tolerant primary amine membranes. Comparative data are provided using BSA and DNA as benchmark biomolecules. In addition, data from leading biopharmaceutical companies featuring actual process feed streams are provided (in full version of paper only, see note at end of article) to demonstrate NatriFlo HD-Q’s scalability and its performance for clearing HCPs, viruses, and DNA from MAb solutions.
Summary of Findings
NatriFlo HD-Q membranes demonstrate the following performance advantages for polishing chromatography operations:
Improved Salt Tolerance and Wider Buffer Compatibility:The membranes demonstrated superior dynamic binding capacity in the presence of salt or phosphate background.
High Loading Capacity without Compromising Process Robustness: Results show excellent virus (xMuLV and MMV) and DNA clearance from partially purified MAb samples for loads as high as 10 kg/L over a broad design space. The membranes also demonstrated excellent HCP clearance with no sign of increasing breakthrough even at 10 kg/L load.
Simple Scale-Up: The membranes provide excellent and consistent HCP clearance from laboratory to process scale.
Truly Single-Use, Plug-and-Play Format: There is no column packing, qualification, cleaning, or validation and no risk of cross contamination.
Overview of Study
This study compares the dynamic BSA binding capacity of NatriFlo HD-Q membrane adsorbers with commonly used alternative technologies. Results demonstrate performance in challenging process conditions (salt and phosphate background).
Salt Tolerance
To evaluate salt tolerance, a feed stream (1 g/L BSA in 25 mM Tris + NaCl buffer, pH 8.0) was processed at a variety of salt concentrations, ranging from 0 to 125 mM NaCl. The high salt tolerance of the NatriFlo HD-Q is evident in Figure 1, where the material maintained superior dynamic binding capacity over a wide range of conductivities in comparison with all membrane adsorbers, including salt-tolerant media. Resin 1Q demonstrated similar binding capacity as NatriFlo HD-Q membranes but suffers from throughput limitations inherent to column chromatography.
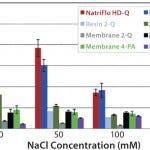
FIGURE 1: 10% BT BSA binding capacity: salt tolerance.
The typical residence time for any column at process scale is at least two minutes, which constrains the loading capacity of a column. For example, a typical column would need more than 66 hours just to load a feed sample having 5 g/L titer to 10 kg/L capacity at two minutes of residence time. By comparison, NatriFlo HD-Q membranes would need only 3.3 hours to load the same feed at 10 MV/min flow rate (or 1.3 hours at 25 MV/min flow rate for the same impurities clearance performance).
NatriFlo HD-Q membranes offer superior dynamic binding capacity over a wide conductivity range without any throughput constraints. The high salt tolerance of Natrix HD-Q membranes provides greater flexibility during process design. In addition, NatriFlo HD-Q membranes can achieve high levels of performance without the need to dilute salt concentrations to reduced conductivity.
Phosphate Tolerance
Phosphate is one of the most popular and widely used buffers in the 5.88.0 pH range. Natrix HD-Q membranes can tolerate the presence of phosphate ions unlike other salt-tolerant anion-exchange membrane adsorbers, which can be used only with monovalent buffers. Figure 2 compares phosphate tolerance of the NatriFlo HD-Q membrane with alternative technologies. All the alternative devices were run as per the instructions provided by the vendors. All membrane adsorbers were run at 10 MV/min flow rate, and the 1-mL prepacked columns were run at 1 CV/min as recommended by the supplier. The feed sample (1 g/L BSA, pH 8.0) was prepared in phosphate buffers having concentration from 25 to 75 mM.
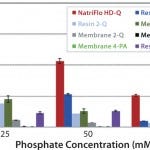
FIGURE 2: 10% BT BSA binding capacity: phosphate tolerance.
Figure 2 clearly shows superior dynamic binding capacity of NatriFlo HD-Q over competitive technologies in phosphate buffer. The salt-tolerant membrane with primary amine-based chemistry did not bind BSA from phosphate buffer, whereas Natrix HD-Q membranes achieved dynamic BSA binding capacity as high as 186 mg/mL at 10% breakthrough in the presence of 25 mM phosphate at pH 8.
Overall, the NatriFlo HD-Q dynamic binding capacity was more than 50% higher than the next best alternative column chromatography. This advantage was most pronounced at higher phosphate concentrations.
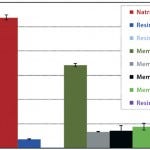
FIGURE 3: 10% BT DNA binding capacity.
DNA Binding Capacity
Dynamic DNA binding capacity was tested by processing a feed stream (0.1 g/L herring testes DNA in 25 mM Tris buffer, pH 8.0) through the various devices. The average DNA dynamic binding capacity of the NatriFlo HD-Q was 27 mg/mL, 59% higher than the next closest alternative (membrane 1-Q at 17 mg/mL). None of the other devices exceeded 5 mg/mL.
The Natrix HD-Q membrane was the only alternative to show excellent binding capacity for both protein and DNA without throughput limitation. This can be explained by the very open structure and easily accessible high ligand density in the Natrix HD membrane.
This article summarizes a paper published by Natrix Separations. The full version can be downloaded at: http://natrixseparations.com/natriflo_paper.
James Stout, PhD, is vice president, process sciences, at Natrix Separations, 5295 John Lucas Drive, Unit 6, Burlington, Ontario, L7L 6A8 Canada, [email protected]; US and Canada :1-866-990-6972, International: 1-905-319-2682.
You May Also Like






