March 2016 Supplement
Adoption of single-use manufacturing continues to expand globally and is showing no signs of slowing down. And why should it? The biopharmaceutical industry is challenged to produce safe, effective therapies and vaccines amid the constant pressure to lower cost per dose in addressing healthcare needs of not only the western hemisphere, but emerging markets as well. To meet these challenges, manufacturers are turning to single-use and hybrid systems that incorporate a balance of stainless and single-use equipment. Over the past decade, single use has grown from an upstream tool used mainly by biotechnology companies in early stage drug development and experimentation (allowing them to fail fast) into a broadly deployed downstream therapeutic and vaccine manufacturing platform. Single-use technology is being adopted by drug manufacturers around the globe.
In response to the deadly Ebola outbreak in West Africa during 2014, industry leaders GlaxoSmithKline and Merck & Company used disposables as a pivotal pa...
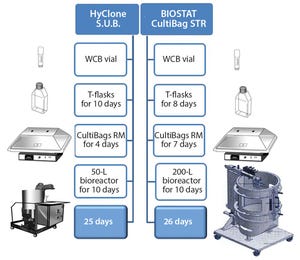
Figure 1: Upstream rh-FSH process with two scales of single-use bioreactor systems
Follicle-stimulating hormone (FSH) is a heterodimeric glycoprotein consisting of noncovalently linked α and β subunits. It stimulates the growth of immature follicles in ovaries and primary spermatocytes in testes and thus plays an important role in human reproduction (
1
). Human menopausal gonadotropin for infertility treatment was first introduced into clinical practice in 1950 (
2
,
3
). Subsequently, treatments with urinary FSH have been replaced by recombinant human FSH (rh-FSH), which has been shown to provide several advantages such as absence of luteinizing hormone activity, higher specific activity, and lower risk of infection (
4
).
The first commercially marketed rh-FSH was developed using a stainless steel vessel and a continuous perfusion system with serum-containing media (
5
). However, many biopharmaceutical companies are switching from such production systems to single-use (SU) bioreactors (
6
). That has...
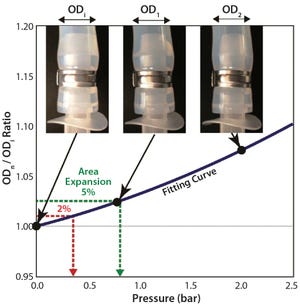
Figure 1: Swelling by internal pressure for tubing 2 (3/8-in. ID); calculation of swelling pressure from tube-swelling curve fitted by second-order polynomial fitting
Single-use systems (SUSs) are engineered process equipment solutions for pharmaceutical and biologics production. They offer several key advantages, such as lowering energy costs to reduce utility requirements, minimizing cleaning validation efforts, reducing water and chemical use, and enabling flexibility in manufacturing. Use of SUSs is increasingly popular in almost all fields of bioprocess applications (
1
,
2
).
SUSs most commonly comprise components made of polymeric materials, which together create a system or unit operation designed for one-time or campaign use. Single-use assemblies are self-contained and preassembled plastic fluid paths, usually gamma irradiated for sterility and ready-to-use, built from a combination of standard components. Single-use assemblies can be customized to meet defined applications and unit operations....
Table 1: Technical guidance summary
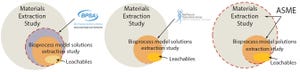
Implementation of single-use systems in both upstream and downstream applications continues to grow rapidly. Parallel to that growth is the concern about purity levels of polymeric single-use systems because compounds found in disposable materials of construction can leach into process fluids or final drug products.
By definition, extractables studies are intended to identify chemical substances that could migrate into process fluids. These tests generally take place under exaggerated conditions that exceed those typically found in bioprocess manufacturing or storage. Industry organizations such as the Bio-Process Systems Alliance (BPSA), the American Society of Mechanical Engineers–Bioprocessing Equipment (ASME BPE), and the BioPhorum Operations Group (BPOG) have established extractables testing guidelines and protocols that can be categorized as either material-specific or bioprocess-specific worstcase testing.
The actual protocol used in an extractables evaluation is...
Photo 1: Original disposable Aber Instruments biomass probe attached to light-weight preamplifier
Often simply referred to as
capacitance
, radio-frequency (RF) impedance has been used for over two decades to measure online biomass. It is generally regarded as the most robust and reliable method to monitor live-cell concentrations in mammalian cell culture (
1
). Many biopharmaceutical companies have now made the transition from conventional glass or stainless steel multiuse (MU) vessels to single-use (SU) bioreactors. Disposables are rapidly becoming the preferred platform for new processes requiring current good manufacturing practice (CGMP) compliance. At the process development level, RF impedance typically is used in the majority of MU bioreactors to optimize process cell growth. So it is important that the same technology be available in CGMP production bioreactors. Here we show how a new capacitance-based biomass probe has been developed for SU bioreactors with applications for the technology in d...
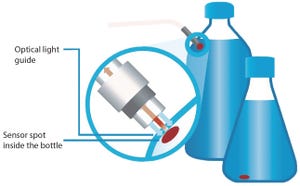
Figure 1: Contact-free measurement with chemical optical sensors
Over the past decade, the application of chemical optical sensors for bioprocess monitoring has gradually taken roots. Constant further development of this measurement technology and the possibility to manufacture such sensors in various designs (even for single-use applications) have led to new state-of-the-art devices for the biotechnology sector. Chemical optical sensors enable in situ, real-time monitoring of important culture parameters without sampling and therefore without disturbing a culture. Implementing this technology can decrease workloads and deepen knowledge about bioprocesses.
In many culture environments and vessel formats, application of electrodes would be very cumbersome or impossible. Too many electrodes disturb the flow pattern of some vessels (especially at small scale) or are simply difficult to integrate because of a lack of space. Such difficulties can be overcome by miniaturized chemical optical sensors. Their smal...
SARTORIUS STEDIM BIOTECH (WWW.SARTORIUS.COM)
In biopharmaceutical development and manufacturing processes, single-use technology has become widely accepted (
1
). Storage and cultivation bags are particularly common. They are fabricated from plastics consisting of multilayer films and are typically provided gamma-sterilized by suppliers (
2
). The bags offer several advantages such as savings in time and cost. Lowered contamination risk results from reduced cleaning and sterilization demands. However, some adverse effects of polymer films on cell growth and metabolism have been reported, both for storage and cultivation bags with polyethylene contact layers (
3
,
4
).
The most serious adverse effects are caused by leachable components generated in the complex bag manufacturing process, particularly during film extrusion and gamma sterilization (
5
,
6
). Polymers and additives (antioxidants, plasticizers, or slip agents) can degrade as a consequence of the intense energetic treatment (high temperatures ...