How Advanced Single-Use Polymeric Materials Influence Extractables TestingHow Advanced Single-Use Polymeric Materials Influence Extractables Testing
March 14, 2016

Table 1: Technical guidance summary
Implementation of single-use systems in both upstream and downstream applications continues to grow rapidly. Parallel to that growth is the concern about purity levels of polymeric single-use systems because compounds found in disposable materials of construction can leach into process fluids or final drug products.
By definition, extractables studies are intended to identify chemical substances that could migrate into process fluids. These tests generally take place under exaggerated conditions that exceed those typically found in bioprocess manufacturing or storage. Industry organizations such as the Bio-Process Systems Alliance (BPSA), the American Society of Mechanical Engineers–Bioprocessing Equipment (ASME BPE), and the BioPhorum Operations Group (BPOG) have established extractables testing guidelines and protocols that can be categorized as either material-specific or bioprocess-specific worstcase testing.
The actual protocol used in an extractables evaluation is often determined according to the intended use of the results (why testing is being performed and what decisions will be made based on the resulting data). Test results may be needed for an initial material screening or used for comparison of materials or competitive products. These studies can also be used as a quality control method or as a substitution for leachable testing.
Another factor that affects extractable testing protocol decisions is technological progress in polymers used in single-use applications. During the early adoption period of single-use systems, the polymers used as materials of construction were typically commodity polymers. They serviced multiple applications across multiple industries. Unfortunately, the amounts used in bioprocessing applications were quite small, and the industry was forced to settle with nonapplication-specific materials. Fortunately, the implementation of single-use systems (SUS) has increased to a point at which demand for better polymer performance has grown, suppliers are moving toward advanced polymeric materials. Such materials can be formulated and controlled specifically for the needs of bioprocessing, or they can be adopted from other high-technology industries with similar needs for high purity and performance.
Industry Guidance
Many resources can be referenced for extractables testing guidance. The range of available resources starts with industry communities such as BPSA and BPOG, who provide best-practices documents and guidelines. Professional groups such as ASME, American Society for Testing and Materials (ASTM), Parenteral Drug Association (PDA), and International Society for Pharmaceutical Engineering (ISPE) provide consensus standards, testing protocols, and technical papers. Finally, compendia and regulatory agencies — e.g., United States Pharmacopeia (USP), European Pharmacopoeia (EP), and the US Food and Drug Administration (FDA) — mandate and enforce requirements to protect the health and safety of the patient population. Looking deeper into a few of the organizations and their guidance on extractable testing provides an interesting perspective.
In the BPSA Technical Guide (1), extractables are described as chemical entities that migrate from process equipment under approved exaggerated conditions. Extraction processes should be performed at higher temperatures, have longer contact times, and use moderately more aggressive solvents than those used in leachables testing. To prevent overpredictions of extractable compounds, conditions of an extractables process should not be overly extreme.
BPOG views extractables testing as a process to rigorously estimate the types and amounts of leachables that will be generated by an SUS component during its intended lifetime. It recommends that extractable exposure times and temperatures represent reasonable worst-case conditions for most typical biomanufacturing applications (2). BPOG further recommends a broad range of buffer-based extraction fluids.
ASME-BPE defines extractables as chemical substances that can be solubilized from polymeric materials using appropriate solvents. Test conditions generally should exceed typical bioprocess manufacturing or storage conditions. ASME-BPE further categorizes the extraction conditions as polymeric/material-specific conditions — using solvents that are aggressive to the particular polymer — and bioprocess-specific conditions, which represent conditions and solvents that are considered extreme for typical bioprocessing applications) (3).
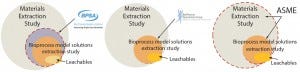
Figure 1: Industry guidance target
Figure 1 shows that recommendations from those three organizations can be further depicted as a series of overlapping circles, where large circles represent extractables detected during material-specific extractables tests, smaller circles represent extractables detected during bioprocess model-solution studies, and ovals represent actual leachables detected using actual process media and process conditions (Figure 1). The challenge that component and system suppliers now face becomes which process or protocol to follow.
Advanced Materials
Similar to the evolution of materials in industries such as medical devices, semiconductors, and automobiles, materials for single-use processing will continue to evolve as the demand for higher performance continues. For example, commodity resins (used for multiple products and applications) are being replaced with advanced materials that are either developed specifically for bioprocessing requirements or adopted from other high-tech industries with similar needs. The physical composition of these materials is such that the testing protocols (as outlined previously) may not provide valuable results. For advanced materials, the typical additives used in commodity resins (e.g., curing agents, catalysts, antioxidants, plasticizers, and release agents) often are present at reduced quantities or may even be eliminated from resin composition. Furthermore, the number of different polymer layers in an assembly may be reduced because one or two advanced materials can meet all the performance requirements of a system, as opposed to five or six materials currently being used in an assembly. Advanced materials also are providing greater chemical inertness and temperature capabilities than their predecessors.
As a result of the material improvements and fewer additives in the resin, the number of possible compounds that could be extracted from such materials is reduced. The improved chemical inertness of advanced materials further reduces the number of compounds solubilized by less-aggressive solvents. And the increased temperature range associated with advanced materials will influence extraction results at moderately higher test temperatures. So testing such advanced materials may be better suited to an aggressive extraction technique. Stronger solvents and higher temperatures would be incorporated to generate a data set of compounds that can be used for a material risk-assessment or quality control process.

Table 2: Case study materials and extraction techniques
Case Studies
In the case studies described below, two different polymer films were exposed to two different extraction techniques to provide a better understanding of how the materials of construction influenced the testing data set. One film was a commercially available polyethylene material frequently used in bioprocessing. The other film was an advanced fluoropolymer material that is frequently used to store, transport, and dispense high-value chemicals and products in the life science and semiconductor industries. The two extraction conditions implemented consisted of a material-specific extraction condition and a bioprocess model solution extraction condition (Table 2).
Results
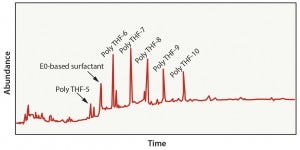
Figure 2: Polyethylene in 50% ethanol extraction results
Case 1: This evaluation represented a bioprocess model solution extraction study in which the interior surface of a polyethylene bag was exposed to 50% ethanol for 21 days at 40 °C. Analytical results detected several oligomers present in the extract solution and an ethylene-oxide–based surfactant (Figure 2). This data set is fairly typical of the analytes that would be expected from polyethylene material using a worst-case bioprocess model solution extraction study. Such information could then be used to execute a risk-assessment process on the material.
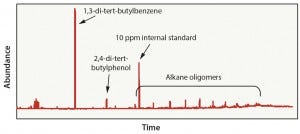
Figure 3: Polyethylene in hexane extraction results
Case 2: This evaluation was representative of a material-specific extraction process in which polyethylene material was exposed to hexane (an aggressive solvent) at a high temperature for a longer time than in Case 1. The objective of this test was to extract as many compounds from the material as possible. This approach led to the detection of 21 organic compounds — including several oligomers, which may be present because of incomplete polymerization of the material or because of degradation of polyethylene during gamma-irradiation exposure (Figure 3). In this case, extraction using 100% hexane is typically inappropriate because of the number of analytes detected that are unlikely to be viable leachables in a final product. This data set could result in unnecessary toxicology evaluations and a longer than normal approval process.
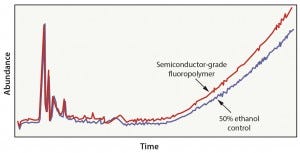
Figure 4: Fluoropolymer in 50% ethanol extraction results
Case 3: This evaluation included an advanced fluoropolymer material that was subjected to a bioprocess model solution extraction protocol in which wetted surfaces were exposed to 50% ethanol for 21 days at 40 °C. In this scenario, the solvent did not extract compounds from the fluoropolymer material (Figure 4). Results can be attributed to the fact that the advanced fluoropolymer material does not contain fillers, additives, or processing agents that are commonly detected in commodity resins (as shown in case 1 and case 2). In this situation, a simulated bioprocess extraction study is of little value, and executing a full-scale bioprocess model-solution extraction study would not be an effective use of time and money.
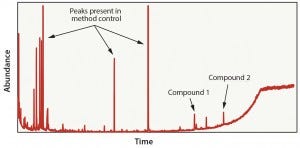
Figure 5: Fluoropolymer in hexane extraction results
Case 4: This evaluation was representative of a material-specific extraction process in which fluoropolymer material was exposed to hexane in a reflux extractor. The test objective was to pull as many compounds out of the material as possible. Because of the composition of the fluoropolymer material, we detected only two compounds (Figure 5). The two compounds were not native to the fluoropolymer material and were likely introduced during the film manufacturing process. Although this test detected only two compounds, it was a more effective way to evaluate the fluoropolymer. Because of the short exposure time and relative low cost, this method also could be leveraged as a quality control process to verify batch consistency of the film.
Understand Extractables Testing
Case studies demonstrated that the type and quantity of compounds detected in an extractable study are not only process dependent, but also material dependent. For polyethylene, a bioprocess model-solution extraction study is likely to be the most effective process to use. Data generated represent what can leach out into actual process fluids, and the study approach can be an effective risk analysis step. An over-aggressive material-specific extraction study with polyethylene can generate an overabundance of compounds — many at levels too low to identify with a high level of confidence and many that have a reduced risk of leaching into final process fluids.
On the other hand, advanced materials such as fluoropolymers that have low or nonexistent levels of fillers, additives, and processing agents are better suited to more aggressive material-specific extraction methods. The bioprocess model solution extraction process may not detect compounds (wasting time and expense), but the aggressive materialspecific extraction method can provide meaningful data for an end-user’s risk assessment and serve as a film quality control metric for suppliers of single-use products.
In all cases, it is important to fully understand the scope of testing, define what decisions will be made based on data, and execute a protocol that is aligned with those goals.
References
1 Martin J et al. BPSA Recommendations for Testing and Evaluation of Extractables from Single-Use. Bio-Process Systems Alliance (BPSA): Washington, DC, 2010.
2 Weibing D, et al. Standardized Extractables Testing Protocol for Single-Use Systems in Biomanufacturing. Pharm. Eng. 34(6) 2014; www.biophorum.com/user_ uploads/extractables-protocol.pdf.
3 ASME BPE-2014 Bioprocessing Equipment. The American Society of Mechanical Engineers: New York, NY, 10 October 2014.
Michael W. Johnson is business development engineering manager at Entegris, Inc.; [email protected].
Entegris®, the Entegris Rings Design® and Creating a Material Advantage® are trademarks of Entegris, Inc. BPSAsm is a service mark of Bio-Process Systems Alliance. ASME® BPE is a registered trademark of The American Society of Mechanical Engineers. BioPhorum® is a registered trademark of Biopharmaceutical Consulting Limited. PDA® is a registered trademark of Parenteral Drug Association. ISPE® is a registered trademark of International Society for Pharmaceutical Engineering. USP® is a registered trademark of United States Pharmacopeia Convention Corporation. ASTM® is a registered trademark of American Society for Testing and Materials Corporation.
You May Also Like






