March 2018 Featured Report
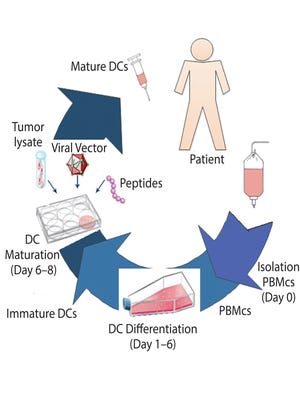
Figure 1: Production of autologous dendritic cells to be used for immunotherapy (DC = dendritic cells, PBMCs = peripheral blood mononuclear cells).
Cell therapies are a growing area of interest for the treatment of a number of indications such as neurological, cardiovascular, and ophthalmological maladies that are refractory to other more conventional drug therapies. A number of cell-based therapy products currently are undergoing clinical trials. The most common target is oncology, which represents 46% of all cell-based therapies through the use of traditional blood-cell and immune-cell–based therapies. For treatment of various cancers, immune cells, lymphocytes (natural killer cells, T cells, and B cells) and dendritic cells (DCs) are typically used, contributing to about half of the oncology-targeted cell-based therapies (
1
).
Cell therapies can be divided into two main groups: those that are administered to the same individual (autologous) or derived from a donor (allogeneic). These groupings dictate...
Photo courtesy of AllCells Donor Center, LeukoLab
The rapidly developing global cell therapy market poses numerous industry challenges for drug development, process scalability, commercialization, and patient safety. The processes of procuring donated human tissue for clinical applications are fraught with many technical, ethical, and legal issues. Allogeneic cell therapies involving primary cell types such as bone marrow mesenchymal stromal/stem cells (BM-MSCs), hematopoietic stem and progenitor cells (HSPCs), and T and natural killer (NK) cells for immunotherapy applications are especially challenging because of the vigorous process of screening and qualifying human tissue donors to prevent transmission of infectious disease and ensure maintenance of an active donor pool. Procurement and safety issues apply regardless of whether primary cell types are used directly to develop cell therapy formulations or as precursor materials to engineer cells for clinical applications (e.g. hiPSC derived cells). In th...
(left to right) Panel host Anne Montgomery, Lee Buckler, Edwin Stone, Jon Rowley, Lynne Frick, and Phil Vanek (PHOTO BY LEAH ROSIN)
After many trials and errors — and milestones — regenerative medicine has become a mainstream part of the biopharmaceutical industry, supported by at least 670 companies and clinics of all sizes. But many experiences in the protein-based industry segment can be leveraged to further improve successful commercialization of advanced therapies. At the 2017 Biotech Week Boston conference,
BioProcess International
editor in chief Anne Montgomery hosted a panel of industry cell therapy experts to discuss key lessons that can be gleaned from the evolution of traditional bioprocessing to support further development of regenerative medicines, to review tools and techniques affecting the current development pathway, and to propose expectations of what may happen over the next decade. Panelists were Jon A. Rowley, founder and chief technology officer of RoosterBio; Lynne Frick, founder...
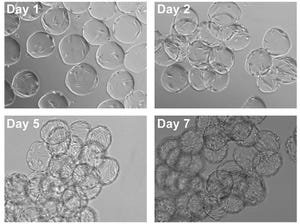
Figure 1: Demonstration of hMSC attachment to Corning Synthemax II–coated dissolvable microcarriers; (A) hMSCs and Synthemax II–coated dissolvable microcarriers during the cell attachment phase at 0 hours (left panel) and 24 hours (right panel). 10× magnification; (B) cell attachment efficiency for hMSCs on Synthemax II-coated dissolvable microcarriers (
n
= 3); cell attachment efficiency was determined by quantifying the number of unattached cells in the culture.
In recent years we have seen an exponential increase in the number of companies testing and validating new regenerative medicine products. Many of these products are reaching late-phase trials with the potential to receive final approval and marketing authorization from regulatory agencies such as the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). In the past several years, we have seen successful launches of regenerative medicine products, including Holoclar (Holostem Advanced Therapies), Kymriah (tisagenlecleuc...