Sourcing Clinical-Grade Human Tissue: Considerations for Supporting Cell Therapy Development and ProductionSourcing Clinical-Grade Human Tissue: Considerations for Supporting Cell Therapy Development and Production
Photo courtesy of AllCells Donor Center, LeukoLab
The rapidly developing global cell therapy market poses numerous industry challenges for drug development, process scalability, commercialization, and patient safety. The processes of procuring donated human tissue for clinical applications are fraught with many technical, ethical, and legal issues. Allogeneic cell therapies involving primary cell types such as bone marrow mesenchymal stromal/stem cells (BM-MSCs), hematopoietic stem and progenitor cells (HSPCs), and T and natural killer (NK) cells for immunotherapy applications are especially challenging because of the vigorous process of screening and qualifying human tissue donors to prevent transmission of infectious disease and ensure maintenance of an active donor pool. Procurement and safety issues apply regardless of whether primary cell types are used directly to develop cell therapy formulations or as precursor materials to engineer cells for clinical applications (e.g. hiPSC derived cells). In this overview, we provide deeper insight into supply-chain considerations for procuring and qualifying human donor tissue as starting material for the generation of clinical cell therapy products. We focus on commercial vendor practices for donor identification, qualification, tissue procurement and handling, and compliance to US regulatory requirements for the ethical treatment of donors.
A Brief Review of the Evolution of Cellular Therapies
The first widely used allogeneic cell therapy originated during WWI with the treatment of wounded soldiers with blood transfusions. The discovery of blood types (A, B, and O) by Karl Landsteiner in 1901 (1) and how they affected patients receiving blood transfusions from unrelated donors enabled physicians to treat patients safely with healthy donor blood. By the mid-1920s, advances in refrigeration and anticoagulants helped establish blood banks that prescreened donors for blood type and enabled storage of collected blood until needed.
In the late 1950s, Dr. E. Donnall Thomas performed the first successful bone marrow transplant between identical twins (2). Discovery of the family of human leukocyte antigens (HLA) and their role in transplant rejection and graft-versus-host disease (GvHD) in the following decades further enabled researchers to refine this procedure and improve it to current standards of HLA matching from allogeneic donors. Identification HSPCs that resided in bone marrow and the ability to produce all blood cells made bone marrow transplants the first stem cell therapy to be routinely used in clinical trials. The next cellular therapies developed used allogeneic keratinocytes and fibroblasts for treating dermal wounds and ulcers and chondrocytes for joint cartilage repair. Those products were manufactured — unlike blood transfusions and bone marrow transplants, which use unmanipulated cells. Cells from donor tissue were isolated and expanded in vitro, sometimes in a biocompatible matrix.
In the past decade, identification of MSCs in the bone marrow with multipotent potential and their immunoregulatory properties led to the use of in vitro expanded MSCs from allogeneic donors to treat autoimmune and inflammatory diseases. Therapeutic value of MSCs can be attributed to
Ability to migrate to sites of inflammation following tissue injury
Ability to differentiate into several mature lineages, including osteoblasts, adipocytes, and chondroblasts
Ability to secrete bioactive molecules capable of stimulating recovery of injured cells and inhibiting inflammation
Ability to perform immunomodulatory functions and a lack of immunogenicity (1).
In 2012, an MSC cell product became the first manufactured stem cell product approved by a regulatory agency for human use: the treatment of steroid refractory GvHD (3). MSCs are now the most common allogeneic cell product being tested in clinical trials (4). Several companies have reported remarkable clinical results from the use of genetically modified, chimeric antigen receptor T (CAR-T) cells targeting CD19+ leukemias, leading to the filing of a biologics license application (BLA) in 2017 (5).
Two recent US-FDA CAR-T approvals of autologous gene therapies are industry milestones, paving the way for a new wave of cell therapy treatments. The first is Kymriah (tisagenlecleucel) for certain pediatric and young adult patients with a form of B-cell acute lymphoblastic leukemia (ALL) that is refractory or has relapsed at least twice. The other is Yescarta (axicabtagene ciloleucel), a cell-based gene therapy to treat adult patients with certain types of large B-cell lymphoma who have not responded to or who have relapsed after at least two other kinds of treatment. Although these cell products are currently autologous, researchers hope to develop allogeneic “off-the-shelf” versions of these T cells to circumvent the need for patient-derived starting material for the manufacturing process. The progress of CAR-T products to the clinic highlight several issues with scaling up the manufacture and sourcing of starting material of an autologous cell product and the advantages of an allogeneic product.
Growing Need for “Clinical-Grade” Human-Source Tissue
Stem and progenitor cells have been used for decades in various cell-based assays for enabling drug discovery and preclinical development. For example, MSCs and CD34+ HSPCs commonly used in cell and gene therapy development can be isolated from tissues such as bone marrow, adipose tissue, peripheral blood, and umbilical cord blood. These multipotent stem cells are commonly isolated through positive or negative selection using different types of cell separation technologies and readily available in fresh and cryopreserved product formats from commercial sources.
Developing cell therapy treatments involving the MSCs, HSPCs, or immune cells (NK or T cells) requires additional screening and qualification of donor source material to address the risk of transmitting infectious disease agents, in compliance with good tissue practices regarding the procurement of human tissues and the Health Insurance Portability and Accountability Act (HIPAA). Demand for such cell types is driven by research and development of new allogeneic cell therapies, and development and validation of bioprocessing protocols for autologous cell and gene therapies.
Federal and State (CA) Regulations |
|---|
Institutional Review Board (IRB) and medical director approvals |
US FDA facility registration |
21 CFR 1271 and associated parts |
Biologics and Tissue Bank licenses (California state) |
Clinical Laboratory Improvement Amendments (CLIA) compliance and certification |
Health Insurance Portability and Accountability Act |
Donor Safety, Screening, and Qualification
Tissue collection facilities are required to comply with state and federal regulations for collection, processing, storage, and distribution of human tissue. A collection facility is responsible for identifying and complying with appropriate regulatory requirements without harming donors.
Donor safety is addressed initially through a federally mandated Institutional Review Board (IRB) approval process. Its primary purpose is to protect the rights, ethical treatment, and welfare of human subjects who donate tissue. For each type of procedure performed at a collection facility, a protocol is written and submitted to the IRB. Contents of the protocol typically include a description of the procedure with disclosure of potential risks to donors, inclusion and exclusion criteria for acceptable donors, required donor screening, and other educational information. Accompanying submission of a protocol to the IRB is an informed consent form (ICF), which must be written so that donors can read and understand it easily. It includes information about what donors are granting permission to have collected, how it will be collected, the amount of time involved, potential side effects, risks and/or discomforts that might occur, confidentiality measures in place by the collection facility, and so on.
From a commercial perspective, donors agree to waive rights of ownership to invention or work products related to their donations. This consent process requires collaboration between the medical director, clinicians, and administrative staff. IRB approval of both the protocol and ICF is required before a collection facility can proceed with donor recruitment. IRB review also must lead to approval of material developed by the collection facility to recruit human donors, including advertising brochures and pamphlets, donor screening and qualification forms, donor education materials, and other relevant information published on the collection facility’s website.
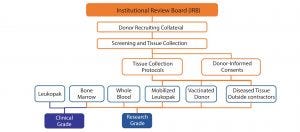
Figure 1: Scope of IRB approvals
In August 2007, the US FDA published Industry Guidelines for Eligibility Determination for Donors of Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps). Together with 21 CFR Part 1271, these documents describe the types of diseases or conditions for which a collection facility must screen for with its donors. Examples include screening by a CLIA-certified laboratory for CMV, HBsAg and HBcAb, HCV, HIV-1 and 2, WNV, RPR, HTLV I and II, Chagas disease, and Zika virus. The scope of viral testing, test methodology used (e.g., antibody based or NAT), and time points for administering viral screens before and after collections (e.g., to detect possible seroconversion of donors) can depend on regulatory requirements within different regulatory jurisdictions. Additional screening of tissue products for human leukocyte antigen (HLA) type, mycoplasma, endotoxins, chromosomal abnormalities by cytogenetic analysis, and total cell count through protocols of the International Society for Hematotherapy and Graft Engineering (ISHAGE) might be required depending on the cell therapy developer’s specific screening and bioprocessing criteria. Figure 1 and the “Regulations” sidebox summarize regulatory considerations.
Maintaining a healthy donor pool is paramount to providing consistently high-quality products that support the growing market demand driven by cell therapy development and commercialization. Because most donors who donate tissue for life science applications participate mainly for financial benefit, it is important that suppliers develop screening techniques to ensure that donors are truthful in reporting their health status. That can be achieved with questionnaires and on-site clinical assessments. Product will not be collected from a donor who does not pass qualifying parameters.
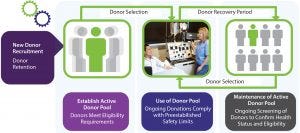
Figure 2: Active donor pool management (photo courtesy of AllCells, LLC)
Completing a Scheduled Tissue Collection: Maintaining an adequate number of eligible donors in a pool ensures that, in the event that a scheduled donor is unable to donate on a particular day, a replacement can be obtained quickly to ensure that product collection proceeds as planned. In some cases, customers choose to have backup donors present on the day of collection because of the criticality of adhering to development and manufacturing timelines. Use and maintenance of an active donor pool requires accurate tracking of individual donations to ensure that donors have adequate time to recover following each donation (Figure 2).
Freshly collected tissue products have limits for defined volumes and frequencies of donations due to safety issues and time frames for viral and blood-count testing. These variables are unique to each collection facility. They are defined using guidelines established by state and federal agencies, professional authorities (such as AABB), along with experience gained through the collection facility’s operations. The facility’s staff expertise is important for ensuring the safety of donors within a clean, friendly, and professional environment for a positive donor experience. Clinical protocols for completing such donations can take from 30 minutes (e.g., whole bone marrow) to four to five hours for a leukapheresis process (e.g., whole Leuko Pak product), or even longer if a collection process involves filgrastim and/or plerixafor-induced cell mobilization of a donor to stimulate egress of HSCs from bone marrow into peripheral blood for subsequent collection.
Smart Procurement: Reducing Risk of Downstream Processing Issues
Cell therapy developers, contract development and manufacturing organizations (CDMOs), and other organizations must be vigilant when performing due diligence to select a supplier that has implemented appropriate operational controls and procedures complying with regulatory requirements in multiple jurisdictions. In practicality, procurement of healthy tissue can be unreliable at times because it depends on human donors and associated time commitments for successfully completing the screening, qualification, and tissue collection processes.
Despite the best planning, clinicians can disqualify an approved healthy donor on a scheduled day of collection because of the donor’s health status (e.g., respiratory infection or unexpected injury), inadequate venous access, or a donor’s inability to tolerate the duration of a full collection procedure. A donor’s personal circumstances, priorities, or schedule can unexpectedly change, resulting in the donor no longer being available on a day of collection. Suppliers should take precautions to reduce the risk of an incomplete collection, which inconveniences or disrupts a cell therapy developer’s manufacturing schedule. A tissue provider must apply effective donor-retention strategies and proactively (and cost effectively) manage its active donor pool to ensure that multiple donors having the right phenotype will be available to support customers’ process scale-up and product commercialization.
Other key challenges associated with the procurement of healthy tissue for allogeneic cell therapy manufacturing include selecting appropriate materials for collection processes, minimizing donor-to-donor variability (which can result in substantially different cellularity profiles and/or quantity of specific cell populations of interest), and maintaining the quality of freshly collected tissue during the testing, packaging, batch release, and delivery process. With industry interest in standardization of source materials and harmonization of regulations across jurisdictions, there will likely be increasing effort to define incoming raw material specifications better and enhance product characterization. Such efforts might include enumeration and/or phenotypic analysis of certain cell types that are clinically valuable for a particular cell therapy application. These challenges are best addressed in a separate, follow-on review of supply chain considerations.
References
1 Karl Landsteiner — Facts. Nobel Media AB 2014. Nobelprize.org. 21 Nov 2017; www.nobelprize.org/nobel_prizes/medicine/laureates/1930/landsteiner-facts.html.
2 E. Donnall Thomas — Facts. Nobel Media AB 2014. Nobelprize.org. 21 Nov 2017; www.nobelprize.org/nobel_prizes/medicine/laureates/1990/thomas-facts.html.
3 Cyranoski D. Canada Approves Stem Cell Product. Nature Biotech. 30, 2012: 571; doi:10.1038/nbt0712-571b.
4 Squillaro T, et al. Clinical Trials with Mesenchymal Stem Cells: An Update. Cell Transplant 25(5) 2016: 829–848.
5 Kymriah (tisagenlecleucel); www.fda.gov/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/ucm573706.htm.
Jay Tong, MD, is founder, president, and CEO; corresponding author Wayne Vaz is vice president of corporate development; Lewis Chapman was formerly vice president of global commercial operations; Timothy Fong, PhD, is vice president of science and biotechnology; Afshin Shirazian is senior manager of marketing; James Kyu Lee is associate director of global commercial operations; Sheila Smith is associate director of clinical operations; and Qin Chen, PhD, is senior global commercial applications scientist, all at AllCells, LLC, 1301 Harbor Bay Parkway, Ste. 200, Alameda, CA 94502; 1-510-671-8676; [email protected].
You May Also Like






