May 2017 Featured Report
With product portfolios that cover both the upstream (production) and downstream (processing) aspects of biopharmaceutical manufacturing, large single-use technology suppliers such as GE Healthcare can address questions of integration and continuous processing with a holistic perspective. (WWW.GELIFESCIENCES.COM)
Despite decades of advancement in characterization analytics, biotherapeutics still are largely defined by the manufacturing processes used to make them. This linking of process to clinical results (and thus to commercial success) has made the biopharmaceutical industry somewhat risk-averse when it comes to the adoption of new technologies. That desire to “derisk” biomanufacturing through better process understanding — as well as the need to adapt to uncertainties in patient population size through process flexibility — in turn drives the need for adoption of new technologies.
Case in point: continuous processing. It’s pretty much a “given” in many other industries — such as those making chemical...
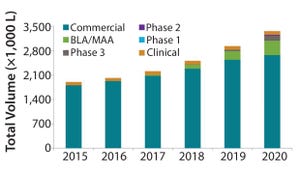
Figure 1: Industry-wide demand forecast for mammalian cell culture capacity (bioTRAK database and forecasting model)
The biopharmaceutical industry is adding mammalian cell culture capacity at rates that we haven’t seen in over a decade. Over the past five years (2012–2016), we estimate that industry-wide capacity has increased from 3.4 ML to 4.0 ML, an increase of 18% (
1
). We estimate that industry-wide capacity will increase over the coming five years (2016–2020) to 5.7 ML, an increase of >40%. Clearly, this growth is a response to the continued increase in demand for biopharmaceutical products and to the current tightening capacity environment.
Biomanufacturers also have announced a diversity of facilities over the years, with a mix of both conventional “six-pack” large stainless steel (SS) facilities and large commercial single-use (SU) “facility-of-the-future” projects. Perhaps it is time to acknowledge that both types of facilities have a significant role to play in supplying modern biopharmaceuti...
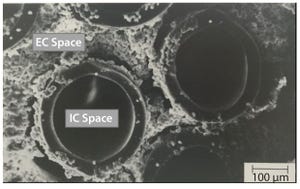
Figure 1: AcuSyst hollow-fiber bioreactors; electron microscopy images of cells growing around hollow fibers within an AcuSyst bioreactor; cells grow to high densities in the extra capillary (EC) space while media flow through the intracapillary (IC) space. These bioreactors scale linearly by adding hollow-fiber cartridges in parallel.
Recent advances in protein engineering have identified new classes of complex biotherapeutics that challenge existing manufacturing platforms. These products have unique cell culture requirements that make them difficult to manufacture cost effectively. Industry standard bioprocessing platforms include large-scale (1,000–5,000 L) batch and fed-batch stirred-tank bioreactors. Historically, the powerhouse molecule of the biologics industry has been human IgG, which necessitates those large-scale platforms. Difficult-to-express proteins and other new modalities (including precision medicine and orphan drugs) have increased pressure on manufacturers to produce smaller batch siz...
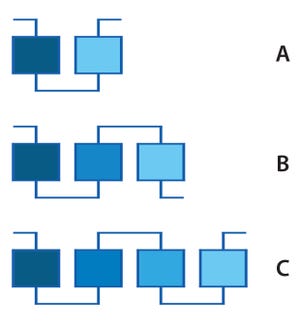
Figure 1: In-line dilution
Over the past 30 years, several biopharmaceuticals have been produced by continuous cell culture processes run in a chemostat or perfusion mode. In most cases, no alternative was available to produce certain unstable molecules (
1
). However, downstream processing is and has remained a step-by-step batch operation. Continuous processing generally requires more process knowledge, equipment, and technological advances than do batch processes.
With the maturity of bioprocessing and increasing awareness of manufacturing costs, companies are focusing on developing continuous downstream processing (CDSP). A 2007 cost study concluded that switching from batch processing to CDSP of monoclonal antibodies (MAbs) could reduce operational costs by almost 70%, a very promising means of increasing production chain economy (
2
).
Motivations for the companies to evaluate CDSP include:
Terminology
Processes can run in several ways. In
fully continuous mode
, each unit operation runs continuous...