May 2020 Featured Report
Networking of manufacturing process control systems can lead to benefits of efficiency, increased productivity, and better facility use, leading to lower cost of goods (CoG). Furthermore, integration of manufacturing systems with manufacturing execution systems (MESs) upward to an enterprise resource planning (ERP) business system improves overall organizational efficiency. An ERP system enables optimization of intrafacility manufacturing resources. For multiple manufacturing facilities, it facilitates optimization across a company’s manufacturing network.
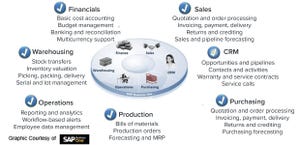
Figure 1: Enterprise resource planning (ERP) system and its functions (CRM = customer relationship management)
Enabling Technologies and Systems
At the enterprise resource planning level (Figure 1), a sales module can monitor incoming orders and forecasts of product demand. A financial module can track cost accounting and impact of fluctuating currencies to optimize profitability. A warehousing module can manage logistics and track available raw materia...
Designing the Right Strategy for Digital Transformation: How a Pragmatic Approach to Digital Transformation Can Help Biomanufacturers Adapt to a Challenging FutureDesigning the Right Strategy for Digital Transformation: How a Pragmatic Approach to Digital Transformation Can Help Biomanufacturers Adapt to a Challenging Future
Figure 1: Database of high-value use cases segmented by value opportunity with key information on each
(QC = quality control)
Although the biopharmaceutical industry has enjoyed explosive growth over the past three decades, it still faces an assortment of challenges. Those include growing portfolio complexities, increased demand volatility, stringent regulatory requirements, increased pricing pressures, and growing technological complexities, all leading to severe pressure on profit margins. To overcome such pressures, biopharmaceutical operations need to become more reliable and agile, and they must realize efficiency gains in both manufacturing and supply chains.
Digital transformation offers strong value opportunities, including a potential for reducing 10–20% of typical conversion costs. Given both the wealth of data available in biotechnology facilities and the resources and capability to invest, biopharmaceutical companies should be well-positioned to benefit from such a transformation, but they exp...
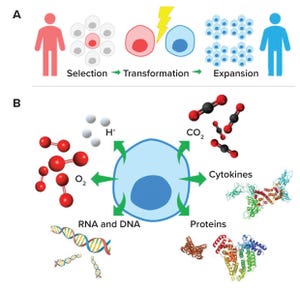
Figure 1: (a) Cell and gene therapy development pathway from patient/donor (red) to recipient (blue); (b) subcellular molecules and ions important for effective cell and gene therapy cultures
Cell and gene therapies are destined to transform the methods by which global healthcare challenges are approached and overcome (
1
). The US Food and Drug Administration is reviewing and approving an increasing number of cell and gene therapy products (
2
), and biopharmaceutical developers are dedicating immense resources to realizing the enormous potential of these therapeutics. Therefore, technologies that facilitate their effective and efficient manufacture will accelerate cell and gene therapies’ transition from medicines of the future to medicines of the present (
3
).
Cell therapies
are administered as live cells from a patient (autologous) or donor (allogeneic) for therapeutic benefit (e.g., blood transfusions and stem-cell therapy).
Gene therapies
can be considered as the addition, silencing, correction,...
Benjamin Bayer developing soft
sensors in MATLAB software
Achieving the high process efficiencies and optimization of Manufacturing 4.0 will require sophisticated software systems, mathematical modeling, and on-line process monitoring. Soft sensors are valuable tools that enable users to measure process parameters in real time. I spoke with Benjamin Bayer, data scientist at Novasign GmbH and doctoral candidate at the University of Natural Resources and Life Sciences in Vienna, Austria, about the potential of soft sensors for bioprocessing and important considerations for their use.
Introduction
How would you describe soft sensors to people who are unfamiliar with them?
The term soft sensor just abbreviates software and sensor. They are used for on-line process monitoring. Hardware measurement devices — typically invasive probes for temperature, pressure, and pH, but also noninvasive sensors such as those for turbidity, spectrometry, or off-gas analysis — all generate unspecific signals. The software part...
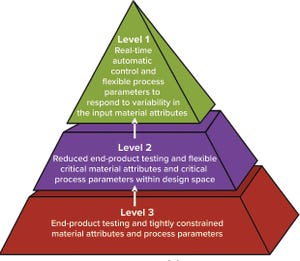
Figure 1: Control strategy implementation levels from traditional (level 3) to enhanced (level 1) approaches (
1
)
Process control enables biomanufacturers to ensure that operating parameters are within defined specifications. A control strategy should be established during early stages of process development while process and product performance are being defined using risk-based methods such as quality by design (QbD) and process analytical technologies (PATs). Confirming process control as an essential part of product development creates greater process knowledge and understanding and provides the first steps toward process optimization. By understanding how process performance relates to product quality, biomanufacturers can reduce time and cost burdens. But implementing control strategies for continuous and integrated processes is not as straightforward as for traditional batch and fed-batch systems, and advanced technologies will be needed to ensure control of an entire continuous process train.
At ...