November-December 2022 Featured Report
On the Cover:
Hepatitis B is an example of a lipid enveloped DNA virus. It is one of the smallest such viruses, with a virion diameter of 42 nm. (
HTTPS://WWW.ALAMY.COM
)
Viral safety in the biopharmaceutical industry is both an upstream-production and downstream-processing concern. Companies must take a multipronged approach using orthogonal methods that are validated to prevent viral contamination or to remove it from biologic drug products. On the upstream side, the focus is on prevention through risk assessment and mitigation. That begins with environmental control of facilities and includes both careful selection of raw materials and cell lines and preparatory filtration of culture media components.
In downstream processing of drug substances, the focus is on inactivation and clearance of viruses that may be present. For inactivating and killing viruses, important technologies include high-temperature, short-term (HT/ST) and solvent/detergent (S/D) treatments of process streams. Virus removal can be...
HTTPS://WWW.ISTOCKPHOTO.COM
The nonionic surfactant Triton X-100 (C
14
H
22
O(C
2
H
4
O)
n
) is a key chemical used in ensuring the viral safety of biological medicinal products. Two pharmaceutical sectors share an extensive historical background with it: biopharmaceuticals and plasma-derived products, for which it is used to inactivate lipid-enveloped viruses. Recently, environmental regulations in the European Union have encouraged or mandated a phase-out of this surfactant (
1
). The goal of the ruling is to protect aquatic ecosystems from potential Triton X-100 degradation products that can function as endocrine disruptors with estrogen-mimetic activity. The phase-out will affect medicinal products for European markets, depending on approval status and the point of manufacture at which they enter the European Union. Below, we discuss the potential consequences of those regulations on both key industry sectors.
For Plasma-Derived Products
Solvent/detergent (S/D) inactivation is the industry-standard un...
As described in ICH Q5A on virus safety of biotechnological products (
1
) and the European guideline on virus safety of biotechnological products, EMEA 398498 (
2
), viral clearance studies are mandated as part of the viral safety evaluation of products derived from human or other mammalian cell lines. When acceptable ranges of process parameters are known, both guidelines recommend that scale-down models be evaluated under worst-case conditions for viral clearance.
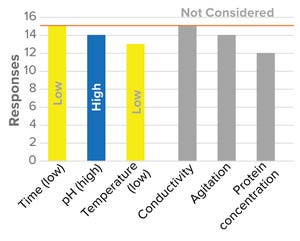
The BioPhorum Development Group’s viral clearance workstream performed a benchmarking survey to identify operating parameters that commonly are considered to present worst-case conditions for viral clearance. The survey further assessed whether companies evaluated process parameters differently for clinical trial stages (investigational new drug applications, INDs) and for commercialization (biologics license applications, BLAs).
Worst-Case Conditions
Worst-case conditions for a viral clearance step differ depending on its stage in a manufact...
Minute virus of mice (MVM) was the model virus used for this study.
(
HTTPS://COMMONS.WIKIMEDIA.ORG
).
Monoclonal antibody (MAb) and other therapeutic biologics produced by mammalian cells have the potential to introduce endogenous retroviruses and can be infected with adventitious viruses through raw materials or other parts of the biomanufacturing process (
1–3
). Based on regulatory guidelines, products derived from mammalian cells must contain less than one virus particle per million doses, which requires purification processes to demonstrate virus removal capabilities of about 12–18 log
10
clearance of endogenous retroviruses and 6 log
10
clearance for adventitious viruses (
4
).
Orthogonal virus clearance steps in typical downstream purification processes include low-pH viral inactivation; affinity, ion-exchange (IEX), and hydrophobic-interaction chromatography (HIC) technologies; and nanofiltration (
5
). The latter method is a size-based viral separation step that generally provides >4 log
10
...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.