Preparing for a Virtual AuditPreparing for a Virtual Audit
September 30, 2021
 Audits are a vital quality-management tool of the biopharmaceutical industry. Whether the objective is to verify supplier or partner qualifications, contribute to corrective and preventative actions (CAPA), or fulfill regulatory compliance requirements, conducting proactive auditing is key to successful operations.
Audits are a vital quality-management tool of the biopharmaceutical industry. Whether the objective is to verify supplier or partner qualifications, contribute to corrective and preventative actions (CAPA), or fulfill regulatory compliance requirements, conducting proactive auditing is key to successful operations.
Over the past year, virtual audits —also known as remote or distance audits — have enabled biopharmaceutical companies to meet compliance and quality assurance demands despite COVID-19–related travel restrictions and social-distancing protocols. Notwithstanding the challenges of virtual audits, the benefits that they provide are likely to make them a long-term practice.
Developing an effective strategy can help biopharmaceutical companies successfully navigate virtual audits. Such a strategy should begin with a risk-based evaluation of facility or supplier quality and remote-audit readiness. Next, a company should develop its complete and comprehensive plan for updating corporate procedures to cover virtual processes, including a remote agenda and information and communications technologies (ICT) options and support.
Document access, security, and confidentiality are important considerations and the best approach for providing a comprehensive tour from a distance. An effective model for a virtual audit workflow will combine those elements and include subsequent reporting and follow-up procedures.
Evolution of the Virtual Audit and Why It’s Here to Stay
Even before the emergence of COVID-19, a virtual audit offered a way to reduce the time and expense of site-based audits while suitably challenging and checking relevant systems and procedures. Many facilities also had adopted remote internal or external audits to manage those audits that involved high-potency products, aseptic cores, or other areas that might pose a risk to inspectors.
As the pandemic necessitated regional, national, and international lockdowns, on-site audits became not just inconvenient or unsafe, but often impossible. The urgent need for new COVID-19 treatments and vaccines also has made careful quality assurance processes more important than ever.
Postponing audits is impractical. The need for oversight has continued during the pandemic. Timelines for reopening borders and cities for travel are unclear, and rescheduling audits creates a risk of backlogs that could strain resources and create unacceptable delays when site-based visits become possible again.
Embracing the Benefits of Virtual Auditing
Virtual audits are likely to grow in popularity even after the pandemic ends. They offer multiple long-term benefits when compared with site-based audits.
Increased Flexibility: In terms of timing and attendance, virtual audits offer much more flexibility than on-site audits. Multiple stakeholders do not need to juggle schedules to get into the same physical space simultaneously. Issues such as travel delays no longer threaten to derail an audit, and time zones become less prohibitive.
Reduced Costs: Eliminating travel and related lodging and meals can reduce direct expenses and resource use.
Reduced Carbon Footprint: Travel reduction equates to a smaller carbon footprint, thus providing environmental benefits.
Improved Efficiency: Spending less time on site-based audits enables auditors to perform more audits within the same period. Virtual audits reduce the number of visitors to a facility and thus can be less disruptive to operations than on-site audits would be.
Ensuring Success
To take full advantage of those benefits, biopharmaceutical companies must address the possible difficulties of a remote audit. The pandemic has increased many organizations’ adoption of remote ICT and has highlighted the need for robust, reliable information security and protection of confidentiality. With adequate Wi-Fi or broadband access, such capabilities simplify the virtual auditing process. Companies that have not yet implemented such measures (e.g., because they still rely on paper systems, work with older infrastructure, or are in an area without high-speed internet access) will benefit from doing so. When working with small suppliers or others facing such challenges, biopharmaceutical companies might consider providing training on new ICT platforms or providing low-cost, cloud-based ICT services.
Auditors also will require reliable ICT and a suitable remote work environment that minimizes interruptions, noise, and other distractions. As with suppliers and on-site personnel, auditors might need to be trained to work with new systems, platforms, or technologies that are used to facilitate the audit.
Another challenge is ensuring the high quality of objective evidence. After all, the purpose of an audit is to assess and gather such evidence to evaluate the adequacy of system controls and to ensure that a facility or supplier is complying with a biopharmaceutical company’s or regulatory agency’s policies and operational procedures.
To help ensure a successful remote audit, companies will need to consider the best way to relay such evidence to auditors while maintaining security and confidentiality. Consider the following questions:
Can the audit include real-time images?
Can interviews and feedback be recorded?
How can the company ensure that the auditor receives an objective and thorough view of the facility, equipment, operations, and controls?
How can the company provide access to relevant information without compromising privacy or confidentiality?
What type of file sharing is appropriate, and what access levels are needed?
Is the company’s cloud storage plan sufficient for document transfer?
Which documents can be viewed through a camera or online, and which need to be delivered through a courier?
Finding solutions to those problem areas is a key part of planning an effective virtual audit.
Preparation Is Key
The first step in preparing for a virtual audit is to determine whether one is needed. If necessary, biopharmaceutical companies can perform risk assessments (e.g., to evaluate a supplier’s quality history, to prioritize which suppliers need to be audited, and to decide the best way to perform the audit if one is deemed necessary).
Updating Procedures: Another important step — one that should occur before you schedule a virtual audit — is to review and, if necessary, revise corporate standards, site policies, and procedures to ensure that they include information on the proper performance of remote audits. Accurate, updated, and consistent documentation helps both auditors and auditees optimize the efficiency and efficacy of an audit.
Adjust the Agenda: Documentation should include a remote audit agenda, including technology considerations. Begin with the standard site-based agenda, then adapt each item to determine how it can be delivered remotely and which technology will facilitate that transition. Here are some examples:
Video conference calls can replace opening and closing meetings.
Documents can be uploaded to a secure, cloud-based file share to facilitate document review.
Video calls can be used in place of in-person discussions with subject matter experts (SMEs).
On-site facility tours can be replaced by video conferencing —perhaps featuring mixed-reality glasses and a 360° camera.
Be aware that a virtual agenda will need to be more detailed than an on-site agenda. Include the method and timing of completion for each deliverable, schedule specific meeting times with specific people, and include breaks and time for document review.
Indicate Approved ICT Tools
Procedures should specify acceptable options for ICT tools. Look for robust solutions that enable audit objectives in the simplest possible way. Additional but unnecessary options are not worth the frustration or lost productivity that often accompanies a difficult implementation or steep learning curve.
Companies should balance accessibility and security. Data protection and cybersecurity are serious considerations, especially where pharmaceutical intellectual property (IP) is concerned. However, security measures that complicate access to necessary resources can be difficult to enforce.
Specify preferred backups in case of technological troubles. If a company’s preferred video-conferencing tool is Zoom, for example, which alternative platform will be used if that platform is unusable? Which file-sharing software is preferred (and which will serve as an alternative)?
Everyone involved in the implementation, operation, and maintenance of a platform should understand both its functionality and limitations. Questions to consider include the following:
Will images be provided in real-time, or will they be recorded?
Can interviews and meetings be recorded and/or transcribed?
What security features are available, and how is their efficacy verified?
Are there known security vulnerabilities, and how dedicated to security is the platform vendor?
How comprehensive is the platform’s ability to share objective views of facilities, equipment, operations, and controls?
How is relevant information accessed, and how granular are the options to control that access?
What features are available to share documents with the cloud?
What tools are available to guide inspectors through a document in real time?
Similar concerns can arise when verifying the accuracy of provided audit data. Determine how verification will be performed. The planning and documented procedure for that step will need to go into more detail than for site-based audits. Consider the technology that will be used to support that effort. As companies make those decisions, they will need to consider and plan how to overcome technological challenges that their partners, suppliers, and auditors might face. They also should look for platforms with staying power, preferably from vendors that have demonstrated a reliable system of product support.
Sharing and Securing Data
Apart from ensuring that they have the ability to share, review, and verify data, biopharmaceutical companies must consider the regulatory and security implications of doing so. Depending on the sensitivity of documentation, virtual data rooms for secure document sharing can be useful. In such cases, companies will need to determine how much access to provide inspectors and auditors and whether to enable downloads, configure desktop synchronization, and/or limit access times.
Less stringent security needs are likely to be met with a standard cloud-based document-sharing solution such as Microsoft Teams or Box applications. Some platforms (e.g., Teams software) also enable recording and transcription of meetings. Such capabilities can be useful when streamlining the virtual audit process, but they can raise difficulties with surrounding compliance with the General Data Protection Regulation 2016/679 (GDPR) and cybersecurity best practices. Some questions to consider are following:
Is the video conferencing platform encrypted from end to end?
Has a GDPR assessment of the ICT platform been performed?
Have all participants provided consent to be recorded?
Where, how, and how securely are recordings stored, accessed, and exchanged?
What is the process for deleting recordings that are no longer required?
Are platform servers located in or outside the European Union, and what security measures are in place?
What are the risks of third parties gaining access to information that is managed through the ICT platform?
Conducting Facility Tours
Many tools are available for use during comprehensive facility tours. For example, Google Maps can be used to provide a street view of a site. Drones provide options for viewing around and inside the facility. Live streaming with mobile technologies such as a 360° camera can enable more detailed views. Auditors might even be able to review video or recordings from in situ cameras on an operations floor.
Photographs and facility blueprints can offer additional facility details. Emerging technologies can be used to combine approaches for an even more realistic experience. Consider three-dimensional mapping or augmented reality (AR) that overlays computer-aided design (CAD) drawings onto video recorded using a mobile 360° camera or a virtual reality (VR) demonstration of activities that simulate real-world scenarios in a safe, remote environment.
Artificial intelligence (AI) also is expanding the boundaries of possibility for virtual audits. Visual inspection typically includes acceptance criteria for particles and other physical issues with parenteral products, such as damaged primary packaging. Advances in AI are enabling more predictive and prescriptive ways to detect such issues, regardless of the size, color, and position of a particle or flaw. Those approaches might increase productivity and reduce costs and errors. Advanced technologies also could lead to the translation of on-site, in-person visual inspections to automated, continuous monitoring of both good manufacturing practice (GMP) and non-GMP data as part of virtual product quality assurance (1, 2).
Before You Begin
After documenting and preparing for virtual audits, biopharmaceutical companies can follow these tips for a smoother, more efficient experience:
Fully charge all portable equipment before the audit or virtual tour.
If using a smartphone or tablet to support high-quality video connections, turn off notifications.
Avoid virtual private networks (VPNs), which can reduce video quality.
When live streaming, hold the camera steady and in place until the inspector directs otherwise, to improve confidence in the authenticity of the experience.
During the planning stage, define realistic and achievable goals for your chosen technology platforms, especially those that require training.
Schedule regular time for breaks and for participants to log off for document review and other tasks.
Provide personnel training to communicate and answer questions remotely, over live stream, online, or on camera.
Copy and courier documents that might be difficult to read on screen or when suppliers cannot sustain the level of technology to enable remote viewing.
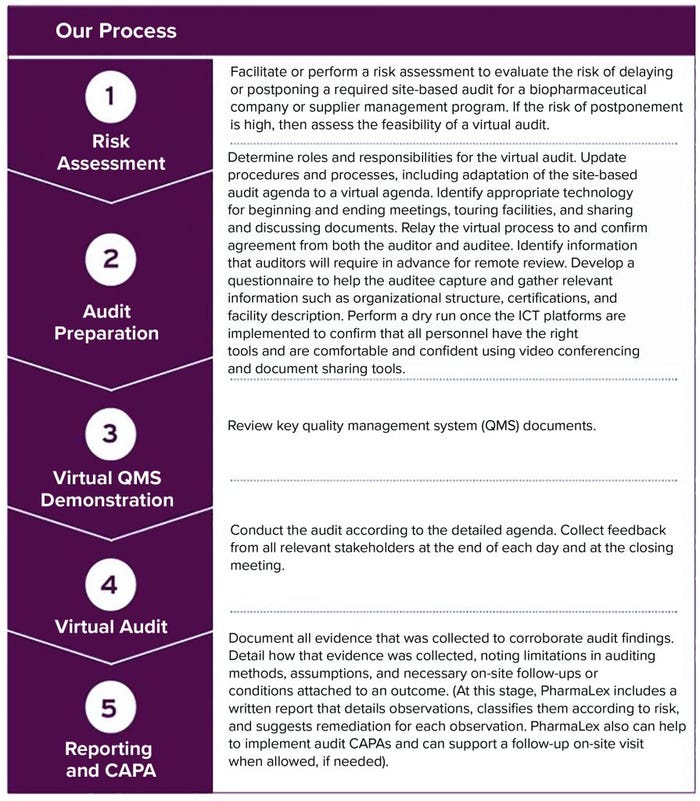
Figure 1: Five-stage process for virtual audit preparation (CAPA = corrective and preventive actions, ICT = information and communications technologies)
A Model for Success
To help biopharmaceutical companies implement successful remote inspections, PharmaLex has developed a virtual auditing model that combines the above elements into an efficient five-stage workflow (Figure 1).
Even after pandemic lockdowns and travel restrictions ease, the use of virtual audits is likely to continue because of the benefits they offer. Biopharmaceutical companies can improve the success of such audits by developing a well-considered plan and virtual-auditing strategy.
The PharmaLex virtual auditing model provides useful guidance for all types of audits, including mock regulatory inspection audits, for suppliers, contract manufacturers, and laboratory organizations. The model also supports internal self-inspection audits for organizations that require additional support.
References
1 Chapman J. How AI Tools Will Transform Quality Management in the Life Sciences. Pharma. Online, 16 April 2016; https://www.pharmaceuticalonline.com/doc/how-ai-tools-will-transform-quality-management-in-the-life-sciences-0001.
2 Krishnamurthy K. AI Is Transforming Industrial Inspection. Innovate UK, 14 February 2019; https://innovateuk.blog.gov.uk/2019/02/14/ai-is-transforming-industrial-inspection.
Kate Coleman is senior director and principal consultant at PharmaLex Ireland, Suite 2, Stafford House, Strand Road, Portmarnock, Dublin D13 H525, Ireland; [email protected]
You May Also Like





