Platform Approaches for the Purification of Antibody FragmentsPlatform Approaches for the Purification of Antibody Fragments

Figure 1: ()
Antibody fragments (e.g., Fab, scFv, DAb, etc.) are set to become the next important class of protein-based biotherapeutics after monoclonal antibodies (MAbs). One of the advantages is that due to their structure and smaller size, antibody fragments possess unique properties (e.g., easier tissue penetration) that suit a range of diagnostic and therapeutic applications.
The industry standard for purifying MAbs is a platform approach using affinity chromatography with protein A as the capture step. The high purification factor and generic conditions associated with this approach have proven particularly attractive to biopharmaceutical manufacturers. Antibody fragments, however, have previously lacked such a platform solution.
With the introduction of Capto L, the first industrial platform for the purification of antibody fragments, is now emerging (Figure 1). With its recombinant protein L ligand, Capto L is a BioProcess™ chromatography medium with a broad range affinity for antibody fragments of different sizes containing kappa light chains.
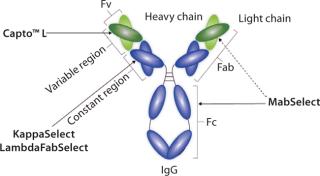
Figure 1: ()
A Three-Step Fab Purification Process Using Capto L
Here we describe an example of using Capto L in a purification process of a kappa subclass Fab originating from an Escherichia coli supernatant. The theoretical pI of the Fab molecule was 8.5, and the molecular weight was 48 kDa. During the set-up of this three-step process, a general workflow was used with the purpose of minimizing the development time. First, different media were screened in a 96-well format using PreDictor™ plates, and wide wash and elution conditions were evaluated. Second, capacity studies were performed in small columns with purified protein (only applicable to binding steps). Third, an elution pH study was performed to find the optimal elution pH. This might be especially important for some molecules that are sensitive to acidic pH values. Design of experiments (DoE) was used to find the best conditions for the step. This general approach also supports quality by design (QbD), where the plates give the characterized space and the DoE in columns render both the design and the control space.
Dynamic binding capacity for Capto L in the capture step was 21 mg/mL at 4 minutes residence time. The elution pH for this Fab was 3.2 when running a gradient from pH 6 to 2.5. Following screening experiments, a three-factor DoE was performed. The studied factors were wash pH, NaCl concentration in the wash, and elution pH. The most important responses in this study were the E. coli protein (ECP) content and the yield. In order to combine the information achieved for ECP and yield and to find the overall optimum, sweetspot analyses can be used.
Figure 2 shows the results of sweetspot analysis for two criteria: an ECP content of 2–30 ppm and a yield of 95–100%. The green surface shows conditions where both criteria were met. A verification run was performed where the wash step contained an acetate buffer with a pH of 5 and 400 mM NaCl. The results were as expected: 96% Fab yield containing only 12 ppm of ECP.
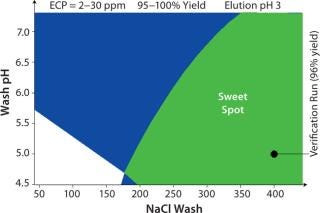
Figure 2: ()
For the second purification step, Capto SP ImpRes (a high-resolution cation exchanger) was chosen due to its ability to efficiently separate aggregates from monomers. A DoE was used where pH, gradient length with NaCl, and load were studied. Some factors (e.g., wash length and final NaCl concentration) were kept constant. Following analyses of monomer yield and aggregate reduction, sweetspot analysis showed a wide experimental space where both the criteria (yield >90% and aggregate content ≤1%) were met.
For the third step, two media were evaluated in plates: Capto Q, an anion exchanger, and Capto adhere, a multimodal anion exchanger. Anion exchangers were included in the process to further reduce contaminant levels. Both Capto Q and Capto adhere are excellent choices for use in flowthrough mode for proteins with a high isoelectric point, such as this Fab. After the plate screening, Capto Q was chosen because no aggregate removal was necessary. Since the isoelectric point is ∼8.5, working at a pH of 8 or less will make the Fab pass in the flowthrough fraction.
Having optimized the three steps and having a better knowledge of the effects of the most significant factors for each step, a process verification was performed.
Figure 3 shows the chromatogram from the capture step with Capto L and the experimental conditions. The Fab eluted in approximately one column volume and had a pH of roughly 4. Tabular results of the verification run for the three-step process are summarized in Table 1.
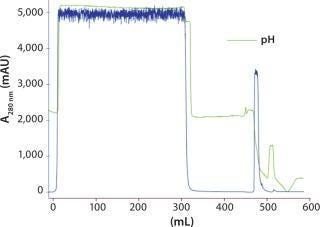
Figure 3: ()
Table 1: Summary results from the verification run; yield ranged 93–97%, giving a total process yield of approximately 87%. Aggregates were reduced from 3.3% to ~0.8%. The ECP in the start sample was more than 400,000 ppm, whereas the final sample contained only 6 ppm. The endotoxin content in the feed was 1.7 million endotoxin units/mg of protein and the final sample was below 0.1 units/mg. The reason for not getting a further endotoxin reduction
between the second and the third steps is that this feasibility study was not carried out in a CGMP facility with the same kind of clean environmental demands. Protein L leakage was below the limit of quantification for all samples.
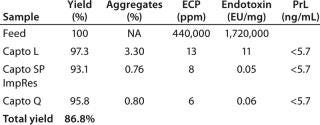
Table 1: Summary results from the verification run; yield ranged 93–97%, giving a total process yield of approximately 87%. Aggregates were reduced from 3.3% to ~0.8%. The ECP in the start sample was more than 400,000 ppm, whereas the final sample contained only 6 ppm. The endotoxin content in the feed was 1.7 million endotoxin units/mg of protein and the final sample was below 0.1 units/mg. The reason for not getting a further endotoxin reduction between the second and the third steps is that this feasibility study was not carried out in a CGMP facility with the same kind of clean environmental demands. Protein L leakage was below the limit of quantification for all samples. ()
Conclusions
Market trends forecast an increased effort in developing therapeutics based on antibody fragments. GE Healthcare has developed Capto L and other chromatography media to enable a platform approach to the purification of antibody fragments. Our results for a Fab process using Capto L in the capture step showed efficient removal of the main contaminants, and a yield of 87% over the complete process.
About the Author
Author Details
Gustav Rodrigo is a senior scientist, and Sara Grönlund is a research engineer at GE Healthcare Life Sciences, Björkgatan 30, 751 84 Uppsala, Sweden; www.gelifesciences.com/bioprocess. GE, GE monogram, and imagination at work are trademarks of General Electric Company. BioProcess, Capto, MabSelect, and PreDictor are trademarks of GE Healthcare companies. © 2012 General Electric Company – all rights reserved.
REFERENCES
1.) GE Healthcare Bio-Sciences AB 2012. Capto L.
2.) GE Healthcare Bio-Sciences AB 2012. Capture Tools for Antibody Fragments.
You May Also Like





