Separation of Monoclonal IgG and Its Aggregates Using TOYOPEARL MX-Trp-650MSeparation of Monoclonal IgG and Its Aggregates Using TOYOPEARL MX-Trp-650M
August 1, 2013

The importance of proper aggregate removal during polishing of a monoclonal antibody (MAb) for therapeutic use is beyond controversy. Severe anaphylactic reactions have been described in the literature for the application of aggregated proteins as a drug byproduct. Traditionally, ion-exchange chromatography (IEX) or hydrophobic-interaction chromatography (HIC) are used to purify a structurally homogeneous product. In case those platforms do not satisfy the requirements for MAb polishing, advanced chromatography resins need to be considered. For instance, mixed-mode stationary phases such as TOYOPEARL MX-Trp-650M may pave the way for more challenging polishing applications. This application note intends to give you some insights into how you could start handling MAb polishing with the tryptophan immobilized ligand.
Screening for the Appropriate Conditions for MAb Aggregate Removal
Mixed-mode chromatography is one approach to combine the advantages of HIC and IEX. The number of potential ligands for mixed-mode chromatography is huge, with potential candidates found in various molecular classes. However, the preferred environment for an antibody restricts the ligand choice. Binding and elution with moderate salt and pH conditions — as well as capacities comparable to IEX — are the focus of ligand selection. Moreover, the need for an appropriate selectivity sets tight bounds. To fulfill these expectations, more complex structures are used than for traditional IEX or HIC ligands. In accordance with this, method development becomes more complex, as well. In case no robotic system is at hand, a straightforward approach for handling the increased number of parameters that affect the process of binding and elution is described in our first example: the polishing of a humanized, monoclonal IgG.
The major factors influencing binding and separation of proteins on TOYOPEARL MX-Trp-650M are the pH and the salt concentration. For a start, three linear-gradient runs will provide hints on the actual working frame for a certain molecule. Figure 1A, Figure 1B, and Figure 1C show three chromatograms of the MAb sample containing ~17% aggregates. Those three runs illustrate a salt gradient (constant pH), a pH gradient (constant salt concentration), and a combined salt and pH gradient. The pH span of the applied chromatofocusing buffer system depends of course on the stability of sample. These buffer systems are either commercially available as ready-to-use buffer systems, or they can be prepared by arranging various (zwitter-)ionic buffer salts with pKs values covering the desired pH span.
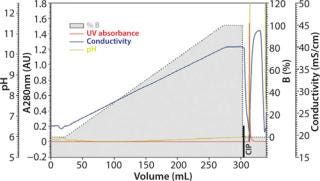
Figure 1a: Column 6.6 mm ID × 2 cm L; mobile phase A buffer pH 4.0 + 0.2 mol/L NaCl; mobile phase B buffer pH 4.0 + 0.5 mol/L NaCl; linear flow 150 cm/h; sample ()
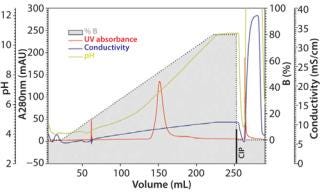
Figure 1b: Column 6.6 mm ID × 2 cm L; mobile phase A buffer pH 4.0 + 0.2 mol/L NaCl; mobile phase B buffer pH 12.0 + 0.2 mol/L NaCL; linear flow 150 cm/h; sample ()
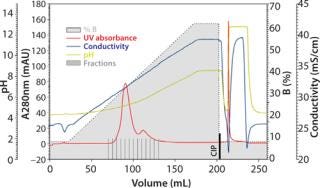
Figure 1c: Column 6.6 mm ID × 2 cm L; mobile phase A buffer pH 4.0 + 0.2 mol/L NaCl; mobile phase B buffer pH 12 + 0.4 mol/L NaCL; linear flow 150 cm/h; sample ()
Although the salt gradient does not allow for protein recovery, the pH gradient leads to the elution of one protein peak. By contrast, the combined pH and salt gradient recovers the protein in two peaks, a monomer peak in the front followed by the aggregates. Quantitative and qualitative analysis of the collected protein peaks was performed by size-exclusion chromatography (SEC) using TSKgel G3000SWXL. Figure 2 presents the corresponding results.
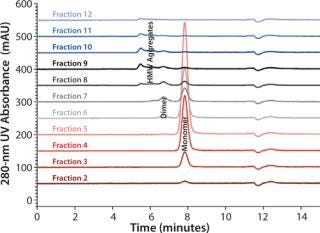
Figure 2: Column TSKgel G3000SWXL 7.8 mm ID × 30 cm L; mobile phase 0.1 mol/L sodium phosphate + 0.1 mol/L sodium sulfate, pH 6.7; flow rate 1 mL/min; detection UV @ 280 nm; sample ()
MAb Aggregate Removal Applying an Up-Scalable Gradient
The screening gradients are rather time consuming and inefficient at process scale due to the covered range of pH 4.0–12.0. Nevertheless, the retrieved results allow for narrowing of the pH and salt concentration range to pH 4.0–6.0 and 0.2–0.4 M NaCl, respectively. This frame can be covered by a sodium acetate buffer (Figure 3). The significantly shortened gradient can be applied for separation, with aggregate content in the monomer pool >1%. Figure 4 presents SEC chromatograms of the collected fractions.
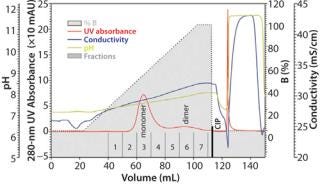
Figure 3: Column 6.6 mm ID × 2 cm L; mobile phase A 0.1 mol/L acetate + 0.2 mol/L NaCl, pH 4.3; mobile phase B 0.1 mol/L acetate + 0.4 mol/L NaCl, pH 5.6; linear flow 150 cm/h; sample ()
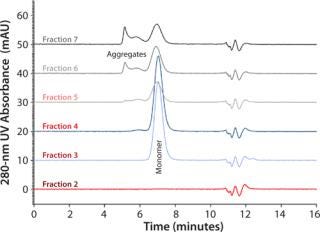
Figure 4: Column TSKgel G3000SWXL 7.8 mm ID × 30 cm L; mobile phase 0.1 mol/L sodium phosphate + 0.1 mol/L sodium sulfate, pH 6.7; flow rate 1 mL/min; detection UV @ 280 nm; sample ()
From these results, we conclude that TOYOPEARL MX-Trp-650M can be used as a highly efficient tool for aggregate removal from MAbs because it offers capacities comparable to IEX along with high recovery and proper selectivity. With the presented screening gradients, it is possible to take advantage of a straightforward method-development approach, which does not necessarily require a robotic system. As a result, method development for mixed-mode chromatography using the tryptophan ligand is no more elaborate than for traditional HIC or IEX resin, although it offers outstanding selectivity for MAbs and MAb aggregates.
For even more challenging separations, users might consider systematic screening for method development to play on the modulation opportunities typically offered by mixed-mode resins. Enabling both procedures with adequate outcome, traditional method development, and advanced systematic robotic screening characterizes TOYOPEARL MX-Trp-650M as a perfect tool for the polishing of MAbs.
About the Author
Author Details
Judith Vajda is a technical specialist at Tosoh Bioscience GmbH Zettachring 6, 70567 Stuttgart, Germany; [email protected].
You May Also Like





