Tangential-Flow Electrophoresis: Investigation of Factors Involved in an Effective Separation of Human Serum Albumin from Human PlasmaTangential-Flow Electrophoresis: Investigation of Factors Involved in an Effective Separation of Human Serum Albumin from Human Plasma
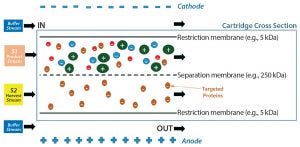
Figure 1: PRIME separation technology uses an electric field and polyacrylamide membranes with different pore sizes to isolate proteins of interest.
Human plasma is a complex mixture of biomolecules such as serum albumin, immunoglobulins, coagulation factors, and others (1). These important protein biomolecules often are present at low levels or lacking in affected patients with certain life-threatening conditions. To extract those valuable proteins, a number of purification methods have been developed over time. Plasma fractionation can be traced back to the middle of the 20th century, when Edwin Cohn of Harvard University developed the first industrial process to purify proteins from crude human plasma (2). The Cohn fractionation method still is widely used today, as is a combination of several column chromatography separations and orthogonal viral inactivation or removal methods, to produce high-quality plasma-derived therapeutics (3).
The Cohn fractionation method uses a protein precipitation approach based on cold ethanol. In this method, different plasma proteins are precipitated by an increasing volume percentage of ethanol (from 0 to 40%) under –5 °C, with a decreasing pH from 7 to <5 (2). Plasma proteins precipitate out in series and thus are separated by filter pressing before further polishing with ion-exchange (IEX) chromatography columns (4). Cohn fractionation requires a large volume of solvents and high energy expenditures for maintaining the subzero processing temperatures, making this method somewhat costly to set up and maintain.
High investment and operating costs thus have restricted most developing countries from establishing their own local fractionation plants to ensure sustainable supplies to local/regional patients. Other costs of transporting and shipping frozen plasma and final finished products further complicate delivery of heat-labile protein products (5). Logistics concerns are easier to manage if a fractionation plant is located closer to patients in regional hospitals and other relevant health institutions. A local fractionation plant also could provide the advantage of curtailing a potential epidemic by providing a supply of purified hyperimmune antibodies in a short period.
Thus, a new plasma fractionation approach based on membrane electrophoresis has been developed, with the goal of providing a practical and cost-effective method to separate proteins from human plasma. This separation technique is known as preparative isolation using membrane electrophoresis (PRIME). Its operating principle is the electrophoretic migration of charged proteins through a polyacrylamide membrane with different pore sizes optimized for a range of biomolecules (Figure 1) (6).
The PRIME process operates based on each protein’s isoelectric point (pI) value. By changing buffering salts and their concentration, you can change the charge of target proteins accordingly by changing their environment. A buffer with a pH greater than that of the protein’s pI negatively charges that protein; a buffer with a pH lower than that of a protein’s pI positively charges that protein; a buffer with the same pH as the pI will neutralize the protein of interest. Therefore, proteins with different pI values can be separated by the PRIME process. Positively charged molecules are attracted toward the negative electrode, whereas negatively charged molecules migrate toward the positive electrode through the pores on the separation membrane. The PRIME process also includes a coseparation based on molecular size by using membranes with predefined or specific molecular weight cutoffs (MWCOs). Two types of membranes are used: restriction membranes and separation membranes.
Using the PRIME system does not involve large consumption of flammable solvents, nor does it require subzero temperatures. Hence, it is safer and more energy efficient than conventional Cohn fractionation used in the blood-plasma industry. With different combinations of pore size (or MWCO values) as well as a buffer system, the system can be applied to isolate a broad range of therapeutic biomolecules.
Studies have shown that the system also can perform a partial virus-/prion-removing step by trapping larger viral/prion particles in the crude feed stream while allowing target therapeutic proteins to cross the separation membrane into harvest (Figure 1) (6). The operating principle here is similar to that of typical nanofiltration.
Note that the PRIME system does not rely on transmembrane pressure for protein transfer. The crossflow or tangential-flow system used during the process can reduce fouling on polyacrylamide membranes in contact with protein–rich complex mixtures (7).
Here, we investigate the effects of several factors on yield and purity of the harvested plasma proteins, including crude feed volume, protein load, feed concentration, and voltage applied.
Figure 2: Process-flow diagrams for extraction of albumin from human plasma using PRIME technology
Materials and Methods
Starting Materials: The key starting materials for our study were human fresh-frozen plasma (FFP) from ADMA BioCenters; boric acid (H3BO3), tris(hydroxymethyl) aminomethane (H2NC(CH2OH)3), and sodium hydroxide (NaOH) from Merck Millipore; and European Pharmacopoeial (EP) human-albumin reference standards (Sigma Aldrich, CAS 70024-90-7). Figure 2 illustrates the PRIME process steps.
Source Plasma: FFP bottles were stored in a –80 °C ultralow-temperature (ULT) freezer. At the start of the process, we removed that from the freezer and placed it in a 2–8 °C cold room for 48 ± 6 hours of partial thawing.
Thawing and Filtration: Then FFP was placed in a 37 °C water bath for about 15 minutes more to ensure that it was completely thawed. Thawed plasma was filtered through 5-µm filters to remove all coarse precipitates.
Diafiltration: FFP was transferred to a Sartorius Sartoflow advanced system for diafiltration. We used a Sartorius PESU 10-kDa membrane for this step, keeping the transmembrane pressure (TMP) at 1.0–1.5 bar throughout the process. The plasma was buffer-exchanged with about 4–7× its volume of Tris-borate buffer (pH 8.9 ± 2), until the final conductivity of the plasma reached ≤1,000 µs/cm. Protein concentration of the FFP remains the same before and after diafiltration. We reduced FFP protein concentration in some experimental runs by diluting it with a corresponding measured amount of Tris-borate buffer (pH 8.9 ± 2).
A PRIME process consists of three stream: the product stream (S1), harvest stream (S2), and the buffer stream. We put diafiltered FFP into S1 and Tris-borate buffer into S2. Maintained at <15 °C, the buffer stream consisting of Tris-borate buffer circulates through the outer sides of the restriction membranes. We used a restriction membrane with a nominal pore size of 5 kDa and a separation membrane with a nominal pore size of 250 kDa, arranging them as shown in Figure 1 and assembled into a cartridge housing. An electrical potential difference of 250 V was applied across that cartridge for six hours, with a harvest collected every 30 minutes. At the end of each harvest, the harvest stream (S2) was collected and replenished with a fresh batch of Tris-borate buffer. All harvests were pooled as a final product at the end of the PRIME process.
Sample Analysis: We analyzed sample concentrations (product yield) using a Bradford assay with a standard calibration curve made using EP human albumin reference standards. We used only narrow concentrations of albumin standard (0–2 mg/mL) to create an accurate standard curve, which we then used to determine the concentration of our samples.
An iMark microplate reader from Bio-Rad Laboratories was used to measure sample absorbances, and sample purity was determined by size-exclusion (SEC) chromatography (quantitative) and reduced sodium-dodecyl sulfate polyacrylamide gel electrophoresis (SDS PAGE) (qualitative). For sample purity analysis, we used a Prominence HPLC system from Shimadzu Scientific with a hydrophilic silica gel column as the stationary phase. EP human albumin and human IgG served as system suitability standards.
We used reduced SDS-PAGE analysis to determine the qualitative purity of our albumin samples. Process yield was determined by dividing the total amount of albumin obtained from the PRIME process by the total amount present before that process. We determined those albumin amounts by multiplying the total amount of protein as measured by the Bradford assay by its corresponding purity as measured by SEC-HPLC.
Results and Discussion
Effect of Feed Volume on the Yield and Purity of Extracted Albumin from Human Plasma: The PRIME process is similar to ultrafiltration/diafiltration (UF/DF), in which membranes of different pore size are used to pass or retain biomolecules of certain molecular sizes. Under ideal conditions, a small area of membrane should be able to handle the maximum amount of feed load in terms of volume and protein mass. We used different volumes of crude feed in our study, processing 15 mL, 60 mL, and 120 mL of FFP under the same operating conditions (protein concentration in feed and harvest duration). All harvest fractions were pooled, and the final yield of the extraction process was calculated according to the following equation:
Process Yield = 100 × (S2 ÷ S1)
where S2 = the total amount of albumin obtained in each harvest (the amount of harvest in the S2 pool) in grams, and S1 = the amount of albumin in the initial feed (S1) in grams.
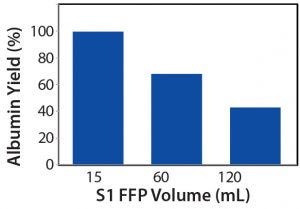
Figure 3: PRIME separation process yield from different volumes of fresh-frozen plasma (FFP) in S1
Figure 3 shows that the FFP volume of 15.0 mL gave the highest process yield (~93.3%), followed by the 60.0-mL (~70.0%) and 120.0-mL (~43.1%) volumes. Because we kept the flow rate of the product, harvest, and buffer streams in the cartridge constant throughout all runs, the FFP would recirculate tangentially across the membrane more frequently with a smaller volume of FFP. Hence, the membrane surface area (17.55 cm2) used could extract the albumin from 15 mL FFP very effectively, as shown by the correspondingly high process yield, although the same membrane surface area might be insufficient for higher feed volumes (e.g., 60.0 mL and 120.0 mL).
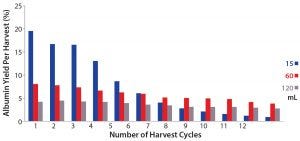
Figure 4: Percentages of albumin extracted from S1 per harvest cycle from different volumes (15 mL, 60 mL, and 120 mL) of S1 crude feed
We also analyzed purity of the harvested albumin with an SEC-HPLC assay (Figure 4). Final harvested albumin from the 15-mL PRIME separation run was 81.7% pure, the 60-mL run was 89.6% pure, and the 120-mL run was 90.7% pure. Hence, by comparing the results in Figures 3, 4, we see that a higher process yield results in lower purity of harvested albumin. Figure 5 shows further that from the first harvest to the twelfth, as more albumin was transferred from S1 to S2 streams, a higher amount of low–molecular-weight (LMW) impurities would be co-extracted — especially after the fourth harvest — as indicated by a decrease in albumin purity in the 15-mL crude-feed run. By contrast, the purity of independent harvest fractions did not differ significantly for the 60-mL and 120-mL runs. That could be attributed to a larger amount of albumin remaining in the higher volumes, so that the impurities could be extracted only after most of that albumin was transferred.
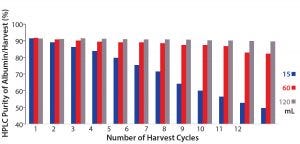
Figure 5: Size-exclusion high-performance liquid chromatography (SEC-HPLC) purity of albumin from each harvest cycle, with different volumes (15 mL, 60 mL, and 120 mL) of S1 crude feed
More albumin remained in the S1 stream after 12 harvests in the 60-mL and 120-mL crude-feed PRIME runs, as indicated by their low process yield. The membrane surface area might be insufficient to accommodate a larger protein load and hence could be saturated, causing a less-effective protein transfer. For the following experiments, we adjusted the protein load with a series of dilutions at different S1 volumes.
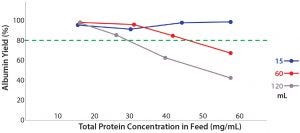
Figure 6: Effects of total protein concentration in different volumes of feed on the PRIME albumin extraction process yield
Effect of Protein Concentration and Mass on the Yield of Albumin Extracted from Human Plasma: Figure 6 shows that with a 15-mL FFP feed volume, the process yield of albumin extraction remained consistently high (>90%) even with a relatively high protein concentration of 51 mg/mL. At the same concentration, larger feed volumes of 60 mL and 120 mL give an albumin yield of 68.0% and 42.9%, respectively. The amount of protein would be higher in larger volumes of the same concentration. Our PRIME unit might require more than 12 harvest cycles to maximize albumin extraction for 60-mL and 120-mL feed volumes. However, for considerations of process economy and buffer consumption, it would be ideal for such a processing unit to achieve a satisfactory protein extraction with a lower amount of buffer consumption and fewer harvest cycles.
However, the membrane surface area might be saturated when a very high protein mass is present in the feed. The membrane pores could be fouled by highly concentrated proteins and thus prevent an effective extraction of protein from the S1 feed stream into the S2 harvest stream. Therefore, to obtain a satisfactory process yield of >80%, a 17.55-cm2 membrane surface area can process a plasma feed with no more than (NMT) 2,202 mg of total protein in a smaller volume of feed (60 mL). That will maximize the recirculation of feed over the membrane surface.
Effect of Voltage on the Yield and Purity of Extracted Albumin from Human Plasma: Because the PRIME process relies on the electrophoretic migration of charged proteins across a polyacrylamide membrane with different pore sizes, the voltage (potential difference) applied across the membrane definitely affects the transfer efficiency.
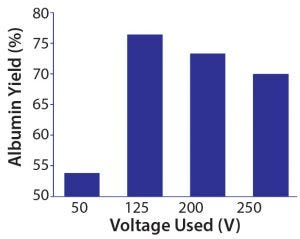
Figure 7: Effects of different voltages used on the overall process yield of albumin, with 60 mL of S1 crude feed and 12 harvests
Figure 7 shows four voltage settings we used in our experiments. The 50-V setting gave the lowest albumin yield (53.8%), whereas 125 V gave the highest albumin yield (76.4%). But with a higher potential difference at 200 V or 250 V, yields were relatively lower at 73.3% and 70.0%, respectively. Experimental results showed that a higher voltage applied did not correlate to a higher process yield. Application of lower voltage might be just sufficient to allow an effective protein migration in crossflow mode. And 200 V and 250 V might have caused very rapid protein transfer, consequently speeding up membrane fouling. Charged proteins could be deposited on the membrane surface and pores more rapidly in a stronger electric field, effectively reducing the surface area and pores available for albumin extraction. The higher potential difference also is undesirable at larger scales because of its relatively higher energy expenditure, which translates into a higher cost of operation.
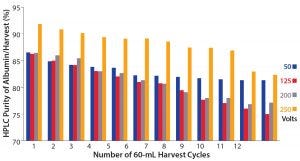
Figure 8: Effects of different voltages used on the purity of extracted albumin in each S2 harvest cycle, with 60 mL of S1 crude feed and a total of 12 harvests
Figure 8 shows the effect of voltage on the purity of the albumin extracted in each harvest cycle. At 250 V, the purity was consistently higher than in harvest fractions obtained with other settings of 50 V, 125 V, or 200 V. That high purity indicates that few impurities were transferred from the S1 stream into the S2 stream. A strong electric field could cause deposition of impurities on the membrane, resulting in a higher-purity albumin harvest at a lower yield (Figure 8).
Note that albumin purity in harvest fractions from 125-V and 200-V processes remained similar in their respective fractions. The PRIME run set to 50 V had the lowest yield (Figure 9), indicating that a relatively lower amount of albumin was extracted successfully from the S1 stream. Purity of albumin harvest dropped through the first six harvests and subsequently stayed relatively consistent throughout the remaining harvest cycles. As Figure 5 shows (in the 15-mL run), albumin generally would be transferred first across the membrane before migration of impurities. Here, the albumin purity in the final four 50-V harvests probably was attributable to a large amount of it left in the S1 stream while most impurities transferred only afterward.
Prime for Optimization
PRIME technology is a relatively new protein purification process that combines elements of UF/DF with electrophoresis to offer a practical alternative to the growing demand for downstream processing of new and challenging biomolecules. We explored the effects of feed volume and potential difference applied on yield and purity. As our results show, a smaller feed volume with a lower protein concentration is advantageous to process yield, but a subsequent increase in coextraction of impurities will decrease product purity.
The amount of protein load that can be processed by a specified membrane surface area unit is limited to a maximum of 170.9 mg/cm2 in protein load for a satisfactory process yield of >80%. A higher potential difference (voltage applied) did not necessary translate into a higher process yield and would indicate larger energy consumption, which is undesirable at manufacturing scale.
This preparative purification system can process high protein concentrations in crude feeds with satisfactory process yields — paired with good purity of a target product in developmental runs as shown herein. In other words, the PRIME unit could achieve high process yields (>90%) under suboptimized conditions. We believe that it is on track to deliver improved results after completion of on-going developmental runs in the near future.
Acknowledgments
This work was supported by (and all scientific data, technical knowledge, and intellectual properties and rights are owned by) PRIME Biologics Pte, Ltd. in Singapore. We acknowledge Lin Jin, Chua Wang Shu, Senthil Kumar, Edgar Lai, Wong Lu Er, and Chinnusamy Murugan for their assistance in our research and writing.
References
1 Cohn EJ, et al. Preparation and Properties of Serum and Plasma Proteins: A System for the Separation into Fractions of the Protein and Lipoprotein Components of Biological Tissues and Fluids. J. Am. Chem. Soc. 68, 1946: 459–475.
2 Pagliardi E, Vitelli A, Gaidano G. Use of the Cohn Method of Fractionation in Clinical Medicine for Analysis of the Protein Fractions of the Sera of Normal Subjects and Those with Blood Protein Disorders. Arch. Sci. Med. (Torino) 104(5) 1957: 605–619.
3 Kistler P, Nitschmann H. Large-Scale Production of Human Plasma Fractions: Eight Years Experience with the Alcohol Fractionation Procedure of Nitschmann, Kistler, and Lergier. Vox Sang. 7, 1962: 414–424.
4 Burnouf T. Modern Plasma Fractionation. Transfus. Med. Rev. 21(2) 2007: 101–117.
5 Farrugia A, Scaramuccia D. The Dynamics of Contract Plasma Fractionation. Biologicals 46, 2017: 159–167.
6 Evtushenko M, et al. Blood Protein Purification and Simultaneous Removal of Nonenveloped Viruses Using Tangential-Flow Preparative Electrophoresis. Electrophoresis 26(1) 2005: 28–34.
7 Lee H, et al. Cleaning Strategies for Flux Recovery of an Ultrafiltration Membrane Fouled By Natural Organic Matter. Water Res. 35(14) 2001: 3301–3308.
Corresponding author Ng Kheng Tee is a quality research consultant, Benjamin Lim is director of operations, Smitha Kenchath is quality manager, and Wong Tee Wee is director of regulatory affairs and quality at PRIME Biologics Pte Ltd., 41 Science Park Road, #01-01C, The Gemini (Lobby B), Singapore 117610; [email protected].
You May Also Like





