The Role of Single-Use Polymeric Solutions in Enabling Cell and Gene Therapy Production - Part 3: Best Practices for Supplier Selection, Qualification, and Validation to Ensure Supply Chain SecurityThe Role of Single-Use Polymeric Solutions in Enabling Cell and Gene Therapy Production - Part 3: Best Practices for Supplier Selection, Qualification, and Validation to Ensure Supply Chain Security
June 12, 2019
Bio-Process Systems Alliance (BPSA) was formed in 2005 as an industry-led international industry association dedicated to encouraging and accelerating the adoption of single-use manufacturing technologies used in the production of biopharmaceuticals and vaccines. Corporate members include plastic equipment suppliers, service providers, and users in the biopharmaceutical industry who share this mission. A key focus of BPSA’s core activities is to educate its members and others through sharing of information and development of best practice guides that help suppliers, users, and regulators to safeguard the quality of drugs produced with single-use technology (SUT).
Part 3 concludes this article series on cell and gene therapy (CGT) manufacturing. Here, the authors focus on ensuring supply chain security and describe strategies for working with suppliers. The information is based largely on experience gathered from the use of these products in blood processing and biologics manufacturing. Differences between those areas and cell therapies are highlighted throughout. (Part 1, Part 2)

(WWW.STOCK.ADOBE.COM)
Factors to Consider First
SUT is used extensively across all stages of biopharmaceutical development and commercial production. Adoption of SUTs has led to significant changes in the way that end users think about supplier selection, qualification, security of supply, and validation. The supply chain for an SUT can be simple or highly complex, thus presenting end users with challenges not inherent in other manufacturing processes.
With a complex supply chain can come increased regulatory scrutiny on supply chain security and risk mitigation strategies in manufacturing. So for companies new to using SUTs for commercial manufacturing, the number of steps and processes involved can seem overwhelming. However, several key points should be considered that can make the entire process more straightforward and less daunting.
Selecting the right supplier may seem to be an easy process. You may say, “Company A has what we need, we’ve used them before, and it works. So we’ll just buy it from them.” This may be a good strategy for procurement of materials for use in small quantities, in a noncritical process, or in an unregulated environment such as a research and developmental laboratory. But this approach cannot be applied to sourcing critical products used in the commercial production of life-saving therapies.
Once a process is defined and a supplier has been selected, that supplier must be validated and continually requalified. Initially, qualification and validation involves a detailed evaluation of many of the same attributes, procedures, and capabilities that form the basis for selection of every supplier. However, it is important to understand that specification, qualification, and validation is a continual process, and that regular supplier meetings and audits are designed to maintain proper quality. BPSA’s Single-Use User Requirements Toolkit Pack, Quality Agreement Template for Single-Use Products, and Information for An Industry Proposal for Change Notification Practices for Single-Use Biomanufacturing Systems provide guidance to ensure that (1, 2).
Qualification and validation include validating manufacturing quality across all processes that a supplier has in place. This includes quality program, product certification, a returns process, paper and site audits, a risk mitigation strategy, a supply chain security program, manufacturing controls, a raw materials sourcing strategy, and a corrective action process. ASTM E3051– 16 (3) explains a structured approach to this task. You should ask the following questions: From a risk management standpoint, does the supplier dual source? Does it make or outsource? Does it have an active continuous improvement process and a new product development program that can support an integrator’s program?
Evaluation and Selection
One framework for supplier evaluation that allows each potential supplier to be evaluated equally and a sourcing decision made based on documented objective evidence is called “The 10 Cs of supplier evaluation and selection” (Table 1). Ten Cs refers to specific attributes or capabilities that all suppliers should be able to demonstrate to potential customers showing that both their immediate and future requirements can be met. Initially developed by DPSS Consultants from the United Kingdom and first published as “The 5 Cs,” the concept has been expanded and is now widely used across multiple industries. If a potential supplier fails to meet a customer’s current and future requirements on more than 30% of the critical attributes in the 10 Cs list, serious consideration should be given to whether that supplier is the right partner for a long-term, sustainable relationship.

Table 1: The 10 Cs of supplier evaluation and selection
One key discussion point in establishing an open and direct relationship with a supplier is to be upfront about the expectations that your company has of its suppliers and how critical those needs are to the success of both businesses. Clear expectations allow both suppliers and end users to benchmark performance, both above and below expectations.
The 11th C for Biomanufacturing
In addition to the 10 Cs, which can be applied to any industry, product supply for biomanufacturing is subject to the 11th C: change control. As part of your supplier selection process, you need to understand both a supplier’s ability to manage changes (e.g., to raw materials, manufacturing processes, or product design) and a supplier’s procedures to mitigate risk associated with your changes as a customer. Questions to review with a supplier could include
What is your change notification policy in terms of time?
What is your right-to-final-buy policy?
What is your change management process?
What are the allowed exceptions to that process?
It is important that suppliers providing SUTs meet their end users’ process requirements and regulatory requirements consistently. A CGT provider must assess the risks associated with trusting a supplier to do that. Determine whether a supplier has appropriate risk mitigation in place relative to the gravity of a situation because patient health partly is in the hands of your suppliers. A mishap that a supplier does not notice could have serious consequences.
Other points should be considered when selecting a supplier. Like the 10 Cs above, these factors not only can directly affect supplier selection, but they also can help manufacturers provide a secure and robust supply chain of critical products needed to commercialize a therapy. Such considerations when selecting a supplier include its use of dual manufacturing and dual sourcing, its disaster recovery plan, and its procedures for qualifying and maintaining its own supply chain security.
For example, questions to ask could include the following:
Does the supplier have a dual manufacturing strategy?
If the supplier has a single production facility, what is its disaster recovery plan in the event of a catastrophic failure?
Does the supplier have a dual-sourcing strategy for critical raw materials?
How does the supplier qualify its suppliers?
Will the supplier share that process with you?
How frequently does the supplier evaluate and audit its own supply chain?
Figure 1 shows some products and raw materials for producing a product as “simple” as a 1.0-L storage bag. However, each SUT product, supply chain, and individual process should be validated and fully traceable to a finished product.
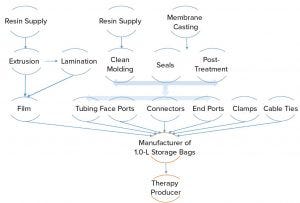
Figure 1: Products and raw materials for manufacture of 1.0-L storage bags
Manufacturing Validation
The final step in supplier evaluation is validation of a supplier’s manufacturing processes. Manufacturing validation extends across all aspects of production operations: from raw materials supply, incoming inspections, operator training validation, facility utilities, and equipment validation. It also includes validation of a supplier’s manufacturing process, including packaging, labeling, and shipping methods. Those are especially critical if a product is fragile, easily broken, or sensitive to temperature and/or humidity. The focus on product quality continues through validation of manufacturing process, facility, equipment, and operators. That process should include the following:
parts and raw materials
primary manufacturing steps (e.g., extrusion, injection molding, rotational molding, vacuum molding, casting)
assembly (e.g., cutting, heat sealing, ultrasonic welding, solvent welding, and manual assembly of components)
personnel
facilities
equipment validation, calibration, and maintenance programs — installation, operation, and performance qualifications (IQ, OQ, and PQ)
environment
finished product.
Validation work with a finished product includes final testing, quality parameters, release criteria, sampling and testing methodology, certification, returns policy, packaging testing and specifications, labeling, and so on.
Collaboration Is Key
To realize the full benefits of SUTs, an unprecedented level of communication and information exchange among system and equipment suppliers and end users is required. More collaboration is needed by biomanufacturers, integrators, component suppliers, and regulators than currently exists with traditional drug manufacturing systems. This increased collaboration must work through all aspects of design, testing, manufacture, and validation of SUTs and the drug products for which they are used. That creates a pathway for the industry to share information and to partner at multiple levels.
References
1 Single-Use User Requirements Toolkit Pack, Quality Agreement Template for Single- Use Products. Bio-Process Systems Alliance; http://bpsalliance.org/technical-guides
2 Information for an Industry Proposal for Change Notification Practices for Single- Use Biomanufacturing Systems. Bio-Process Systems Alliance; http://bpsalliance.org/ technical-guides.
3 ASTM E3051–16: Standard Guide for Specification, Design, Verification, and Application of Single-Use Systems in Pharmaceutical and Biopharmaceutical Manufacturing. ASTM International: West Conshohocken, PA, 2016; doi:10.1520/E3051-16.
For more information, contact Kevin Ott (ottk@ socma.com). This article is published in extended form with permission from Bio-Process Systems Alliance (BPSA), 1400 Crystal Drive Arlington, VA 22202; www.bpsalliance.org. The white paper is available at http://bpsalliance.org/cell-and-genetherapy- resources.
You May Also Like





