The Unican Concept: Engineering Dual Capability into Single-Use VesselsThe Unican Concept: Engineering Dual Capability into Single-Use Vessels
Use of disposable bioreactors in the biopharmaceutical industry has increased gradually over the past several years in pilot, clinical, and production scale facilities (1–4). Reduced time to market in today’s drug industry has created a need for cost-effective development and production strategies as well as manufacturing flexibility. When compared with traditional stainless steel equipment, disposable bioreactor and mixing systems have smaller space requirements, are portable, and come presterilized to eliminate the need for preuse sterilization procedures such as steam-in-place (SIP). Additionally, because their product-contacting surfaces are disposable, the need for clean-in-place (CIP) and associated validation is eliminated (4, 5). Disposable systems also allow for faster turn-around times in between runs, reduce contamination risks, and ultimately increase flexibility for production facilities.
Meanwhile, improved process development and engineering strategies — including optimization of expression-vector elements, more effective clone-selection procedures, and improvements in cell culture media and processes — have increased productivity for cell culture overall. Higher protein expression titers allow for the use of smaller production bioreactors to make the same amount of product and thus increase the applicability of smaller disposable systems (2, 4, 6).
Growing demand for disposable bioreactors and mixers has led to development of many different commercially available options. However, the traditional vendor business model involves locking down future consumable purchases through hardware design. Most single-use bioreactor (SUB) systems use a unique, proprietary, disposable bag design that is housed in a reusable stainless steel support vessel during use.
Commercially available systems differ in many ways: impeller design, agitator location and coupling, instrumentation, working volume, and overall reactor size and geometry. By design, each support vessel works only with its designated bags. In most cases, vendors leverage their intellectual property rights to limit competitors from selling assemblies that would match the specifications of their equipment.
Thus, biopharmaceutical manufacturers are forced to rely on a single supplier for a given vessel’s consumables. This could be detrimental to production should any of a number of challenges arise: e.g., supplier financial/manufacturing troubles, bag quality or technical issues (7–11), incompatibility of supplier single-use components with intended processes, or changes to bag technology (12, 13). Single sourcing presents a major supply chain risk to uninterrupted production, generally avoided whenever possible in good manufacturing practice (GMP) operations, and thus represents a major hurdle in implementation of single-use technology. Based on over 10 years of experience with disposables at Genentech (a member of the Roche group), and having experienced many of those challenges first hand, we view this as the most significant limitation for advancing single-use technologies from a development environment into a GMP setting.
To address the single-sourcing issue, we evaluated several potential solutions, including buying two different disposable bioreactor systems and converting a 200-L SUB into a universal vessel that can support both top- and bottom-mounted single-use bags. For this study, we pursued the latter solution: converting a 200-L vessel from Vendor A into a universal can (“Unican”) that works with single-use bags from Vendor A as well as those from Vendor B. Below we describe mass transfer and cell culture experiments performed to characterize performance with both bags in the same unit.
Industry Survey
First, we surveyed the industry to evaluate its perceptions of the single-sourcing challenge and assess the desire for an alternative solution. End users were asked the following:
Is single sourcing of consumables for a given bioreactor a negative incentive for adopting single-use technology?
Would freedom to source beyond a single hardware provider encourage adoption of single-use systems and thus growth in the single-use supply industry?
Should the end-user community find ways to encourage suppliers to adopt “open platforms” in which different vendors’ consumables and hardware would be interchangeable?
Representatives from seven out of eight companies responded “Yes” to all three questions. The dissenting representative expressed concerns regarding how consumables interchangeability could affect warranties and instrumentation support. That person also questioned whether it would result in end users being wholly responsible for their equipment and expressed concern that it could make investigations more difficult when failures occur.
In other forums — including industry meetings and conference workshops (e.g., Cell Culture Engineering XV and the American Chemical Society’s annual meeting) — attendees have mentioned single-sourcing as a major concern that was impeding implementation of single-use technology. Our industry survey and feedback from different events confirmed that concern over single sourcing for SUBs was common across the industry.
Potential Solutions
The single-sourcing issue could be addressed in a number of ways: e.g., installing two different single-use systems on a production floor, applying a single controller that could operate swappable vessels, using one single-use system with a stainless steel back-up system, developing the ability to roll complete units from different vendors back and forth from storage to a production floor, or accepting a single-sourcing approach and ensuring a multiyear safety stock of consumables to account for vendor-initiated changes or quality issues. Some options would be applicable only to scales of 500 L or less (e.g., rolling 2,000-L vessels around would be problematic).
Based on discussions with colleagues and vendors, we found that one of the most common approaches is to purchase two systems for a production floor. Rather than taking that or any other such approach, we devised an alternative that required hardware modification of a SUB to accommodate bags with different configurations.
Rationale for Not Buying Two Systems: Buying two different systems seems to be a relatively easy option that does not require existing hardware to be modified. However, procuring two different systems for the same purpose is undesirable for GMP operations because it requires validating and maintaining one added system that essentially adds no value. Additionally, constraints on space, finances, and resources are ever present, making it difficult to justify spending for a system that could remain idle as a mere back-up.
Rationale for Modifying Hardware of One System: The Unican approach involves some challenges, including design and execution of hardware modifications. Such modifications could allow vendors to dispute their responsibility for certain types of bag failures during processing when hardware is altered.
However, this option also provides several benefits: a single unit that is compatible with multiple vendor bags, increased flexibility for different cell lines and operating volumes, a single unit to validate (including automation), a standard footprint, better quality and supply chain control, and flexibility to respond to film and supplier issues/changes. Additionally, modifications were made such that it requires under an hour to switch between the two bag systems, and the cost of making those modifications was significantly lower than procuring two systems would have been. Ultimately, the cost of modifying our system into a Unican was <10% of that to purchase a second system.
Materials and Methods
Equipment and Materials: We converted a 200-L SUB from Vendor A with a bottom-mounted agitator into a Unican system. The SUB has four mass flow controllers (Brooks Instrument): three with a maximum flow rate of 20 standard liters/minute (SLPM) for oxygen, air, and nitrogen; one with a maximum flow rate of 5 SLPM for carbon dioxide (CO2). The system is equipped with an open pipe and a microsparger. In our experiments, we used the open pipe as the primary sparger. Temperature in this jacketed SUB is controlled by a temperature control unit (TCU) from Lauda. The TCU has a 7-L fill volume and controls temperature from 0 to 100 °C, which we capped at 50 °C for the purposes of this study. It has a heating power of 2.25 kW and a cooling power of 1.25 kW.
Unican Design: Conceptually, the Unican design could enable use of two, three, or perhaps more different supplier bags with a single stainless steel vessel. However, for this proof-of-concept study, we modified our system to accommodate only two different configurations.
First, we evaluated several different 200-L to 250-L SUBs based on many parameters to determine which system would be easiest to modify. We considered a number of relevant physical characteristics of commercially available SUBs: where agitators and sensors were located, whether the bottom of the vessel was rigid or open, the overall reactor geometry (including chamber diameter, chamber shoulder height, and height/diameter ratio), the number and location of tank baffles, and characteristics of the impeller. Following that assessment, the team decided that it would be easier to fabricate a new top-mounted agitator (rather than a new bottom-mounted agitator) and easier to accommodate a different sensor mechanism in a bottom mounted vessel. This design would be less likely to affect heat transfer or contribute to bag rupture with larger diameter bags (top mounted) in the smaller diameter vessel (bottom mounted) than the inverse.
Based on that assessment, we chose one bottom-mounted SUB (SUB A) for modification. Then we modified it to fit both bottom-mounted single-use bags designed for that system and other bags made for a top-mounted SUB (SUB B).
Several modifications (Figure 1) enabled the use of SUB B bags in the Unican:
adding a top-mounted motor
modifying the top plate used for tubing organization and baffle installation in the traditional SUB A system to make it removable (allowing for adequate headspace when a SUB B bag is in use)
adding a support arm and temperature jackets for SUB B vent filters
removing the bottom portion of the pane that covers the opening in the SUB A vessel to fit SUB B probes
adding a variable-frequency drive (VFD) to control SUB B agitator speed and provide temperature control for the vent-filter jackets
engineering a removable plate to cover the bottom-mounted agitator fitting (used by SUB A bags) while SUB B bags were in use.
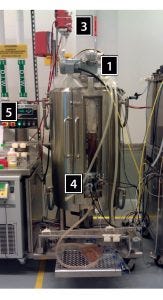 Those modifications were made such that they did not affect the jacketed portion of SUB A’s hardware.
Those modifications were made such that they did not affect the jacketed portion of SUB A’s hardware.

Mass-Transfer Characterization: We conducted mass-transfer studies of both 200-L SUB A and 250-L SUB B bags in the Unican. We measured the volumetric oxygen-transfer coefficient (kLa) at several air-flow rates and agitation speeds using the dynamic gassing-out method (14–16). And we estimated CO2 removal by tracking the rate of solution pH change during sparging.
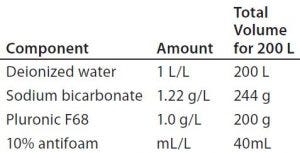
Table 1: kLa test media components
System Preparation: Before beginning mass-transfer studies, we calibrated a 120-mm Finesse EFP K8 electrochemical pH probe using pH 4 and pH 7 buffers. A 12-mm Finesse TruFluor polarographic dissolved oxygen (DO) probe was electronically zeroed and spanned in air. Then we installed both sensors in their designated ports. Next, we mixed media components (Table 1) in an external tank until they were completely dissolved before adding them to the vessel. Air was sparged at 10 SLPM to saturate the medium with oxygen, after which we recalibrated the DO probe for reference at 100% air saturation. Vessel temperature was automatically controlled at 37 °C.
Mass-Transfer Study Procedure: Nitrogen was sparged into the vessel at 20 SLPM. We monitored the DO percentage, collecting data continuously at five-second intervals. Once DO dropped below 30%, we turned off the nitrogen. CO2 was simultaneously sparged into the vessel until pH dropped below 7.0, at which point we turned off the CO2. Next, we set the appropriate agitation speed and sparged air through the primary sparger at an appropriate flow rate for each given test condition: 95, 100, 125, and 140 rpm with respective air-sparge rates of 4, 7, and 10 SLPM at a 200-L working volume in Bag A; agitation speeds of 100, 140, and 174 rpm with respective air-sparge rates of 3, 5, and 10 SLPM at a 200-L working volume in Bag B.

Equation 1 and Equation 2
Mass Transfer Data Analysis: The oxygen transfer rate (OTR) in a cell-free system is defined by Equation 1, where kLa = volumetric oxygen transfer rate per hour; c* = saturated equilibrium DO concentration (%); and c = DO concentration at time t (%). That equation then can be integrated to yield Equation 2.
In this study, we monitored DO continuously at five-second intervals. Using the resulting data, we plotted Equation 2 over time. The slope of the line that was produced is equal to the kLa. In these experiments, kLa was taken to be the slope of the line from 30–90% DO.
CO2 stripping also was characterized in this study. We monitored pH continuously at five-second intervals. Using the resulting data, we plotted the pH change over time. By analyzing the slope of the line between 30% and 90% DO, we could estimate the rate of CO2 stripping as the rate of pH change (pH units/time).
We compared oxygen transfer and CO2 stripping rates obtained in our studies with historical data to evaluate Unican performance in relation to Bags A and B in their respective suppliers’ hardware. Thus we could to determine whether bag performance was compromised by the hardware.
Characterization of Cell Culture Performance: To evaluate the Unican’s performance in cell culture, we used a Chinese hamster ovary (CHO) cell line genetically engineered to secrete a recombinant human antibody. It was derived from a dihydrofolate-reductase deficient DUKX CHO host derived from CHO-K1 cells (17). Based on historical data, we chose this cell line because it demonstrates high cell growth and lactate production followed by a period of lactate consumption. That metabolism can create high oxygen (cell growth) and carbon dioxide (lactate consumption) demands, thus challenging the system to maintain control.
Cell Culture Media: We used chemically defined inoculum train and production media for our cell culture studies. Media were prepared according to standard procedures for the given formulation. We prefiltered all liquid media using an Opticap XL 10 0.22-μm filter (MilliporeSigma) and then sterile-filtered them using an Opticap XL4 0.1-μm filter (MilliporeSigma) before being adding them to a single-use bag.
Our studies also used a chemically defined batch-feed medium according to standard operating procedures (SOPs) for the cell line process. We sterile-filtered the batch-feed media using a Millipak-100 0.1-μm filter (MilliporeSigma).
Cell Culture Conditions: All experiments used a traditional seed-train bioreactor culture in which cells were thawed from a cell bank ampule and then scaled up through a traditional seed-train process. All production-stage cultures were operated in fed-batch mode.
We performed a total of three cell culture experiments: the first and third experiments used Bag B in the Unican system; the second experiment used Bag A in the same system. In all experiments, we maintained DO at a set point of 30% by sparging air and/or oxygen through the primary sparger and controlled pH through the addition of base (Na2CO3) or by sparging CO2 through the primary sparger. Agitation speed was maintained at 125 rpm for all experiments, and we added antifoam as necessary. In fed-batch mode, glucose was added from a stock solution as necessary to prevent glucose deficiency in the cultures.
For all cell culture experiments, we monitored viable cell concentration (VCC), viability, cell size, osmolality, offline pH, and metabolite concentrations daily using a Bioprofile Flex system (Nova Biomedical). To estimate cell growth, we measured percent packed cell volume (%PCV) daily using graduated centrifuge tubes (Kimble Science Products), collecting supernatant samples by centrifuging 5 mL of cell culture fluid for 10 minutes at 830g. Those samples were submitted for titer and product-quality analyses.
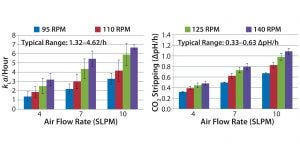
Figure 2: Oxygen transfer and CO2 stripping for Bag A in the Unican; mass transfer and CO2 stripping studies were performed at 200-L working volume. Oxygen transfer and CO2 stripping rates for bioreactors used at Genentech range as listed.
Protein Titer: Protein titer was determined from daily supernatant samples with a high-performance liquid chromatography (HPLC) assay. Results were corrected using the extinction coefficient specific to the antibody molecule.
Product Quality: We determined the relative distribution of charge variants using imaged capillary isoelectric focusing (cIEF) of samples treated with carboxypeptidase. Peak area percentages for acidic, main, and basic regions were identified (18, 19). To evaluate aggregation and fragmentation of the protein, we collected size distribution data using size-exclusion chromatography (20).
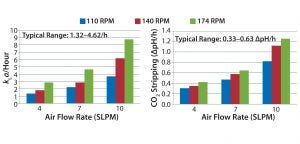
Figure 3: Oxygen transfer and CO2 stripping for Bag B in the Unican; mass transfer and CO2 stripping studies were performed at a 200-L working volume. Oxygen transfer and typical CO2 stripping rates for bioreactors used at Genentech range as listed.
Results and Discussion
Mass transfer rates for Bags A and B (Figures 2 and 3, respectively) of 1.32–4.62/h were within ranges historically observed at Genentech. Similarly, the CO2 stripping rates for Bags A and B (Figures 2 and 3, respectively) of 0.33–0.63 ΔpH/hr were within ranges typically observed at Genentech. At all air flow rates and agitation speeds tested, mass transfer for Bag A in the Unican was comparable with mass transfer of Bag A in its own dedicated hardware (Figure 4). Similarly, mass transfer for Bag B in the Unican was comparable with historical mass transfer data collected using Bag B in its dedicated hardware (Figure 4). This confirmed an absence of mixing, bag folding, or fitting issues that would have caused meaningful changes in mass transfer for the nondedicated hardware.
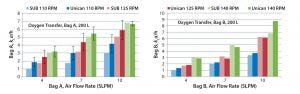
Figure 4: Comparing oxygen transfer in the Unican with standard Bag A and Bag B in their respective single-use bioreactors
Based on those mass transfer data and historical requirements, we decided to maintain the existing gas strategy for Bag B in the Unican, capping the gas flow rate at 10 SLPM. Based on kLa data for Bag A in the Unican (as well as historical studies performed with its dedicated hardware), we capped its flow rates at 7 SLPM. We chose these settings to match mass-transfer performance for both bags in the Unican.

Figure 5: Cell culture performance in Unican — growth; comparing cell culture performance for Bags A and B in the Unican with historical cell culture data comprising four cell culture runs in traditional stainless steel bioreactors
Cell Culture Performance: Figures 5 and 6 show cell culture results. Cell growth for both bags in the Unican was comparable with historical data for the cell line cultured in stainless steel vessels (Figure 5). Metabolite profiles also were similar to control runs (Figure 6). Although the latter results show small variations in lactate and ammonia profiles, both were within the range commonly observed for this cell line host and the platform medium and process conditions.
Protein expression titers were higher for cell culture runs in the Unican (Table 2). All titer, charge, and size-variant data come from day 14 of the cultures, except for charge variant data from Bag A in the Unican obtained on day 12. Product quality (based on charge and size variants) was comparable, with Unican material showing similar or higher percentages of main peak area and similar or lower percentages of product variants — with one exception. Aggregate levels in the Unican were slightly higher for both bags: 2.4 and 2.2 ± 0.03% for Bags A and B in the Unican, respectively, compared with 1.8 ± 0.1% in the controls. Although those levels were slightly higher in the Unican cultures, the results are well within ranges typically observed for this cell line host and these platform medium and process conditions. For that reason, we were not concerned.
Even given the high growth of this cell line, the Unican was able to maintain temperature, pH, and DO control within historic ranges (data not shown) for both bags. Both pH and DO control were comparable across the reactors.

Figure 6: Cell culture performance in Unican — metabolites; comparing cell culture performance for Bags A and B in the Unican with historical cell culture data comprising four cell culture runs in traditional stainless steel bioreactors (center key applies throughout)
An Engineered Solution
We converted a 200-L SUB vessel with a bottom-mounted agitator into a universal vessel (“Unican”) at a fraction of the cost of buying a second system. Our mass-transfer studies demonstrated effective oxygen transfer and CO2 stripping for both top-mounted and bottom-mounted bags in the Unican compared with other large-scale bioreactors used in our company’s network. Similar mass transfer in the Unican and dedicated vessels demonstrate that mixing is not affected by use of Bag B in the Unican. Cell culture profiles (growth, titer, product quality) were comparable with historical data. Product-quality results also were consistent for either bag configuration in the Unican hardware. Although aggregate levels were slightly higher in the Unican than in control runs, the results were well within ranges typically observed for the cell line host and platform media and process conditions, thus were not concerning to the team.
Modifications did not affect the jacketed part of the hardware, and changeover from using Bag A to Bag B takes under an hour. The Unican system provides flexibility to mitigate the risk of bag/film changes or problems and allows for dual sourcing of consumables for disposable bioreactors. Genentech’s cell culture pilot plant currently uses the Unican system routinely.
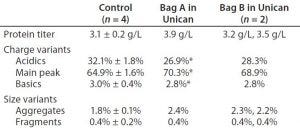
Table 2: Comparing end-of-run product titer, charge- and size-variant data for both bags in the Unican with historical data for the cell line (Control); for “Control” and “Bag B in Unican,” numbers are represented as the average ± 1 SD. Samples marked * come from day 12 of the culture; all other samples come from day 14. Charge variant data were available for only one of the two Unican runs using Bag B.
This proof-of-concept study focused on modification of a bioreactor, but a similar concept could be applied to single-use mixing vessels as well. Because of industry interest, we have shared design concepts with end users who were interested in fabricating and modifying their existing systems. However, we encourage vendors to recognize the industry’s interests and needs and offer off-the-shelf mixing and bioreactor systems that can operate with bags from different vendors. As single-use technology innovation and adoption continues to evolve and new challenges are uncovered, collaboration between end users and vendors will be increasingly critical to responding to a range of technical, quality, and supply chain issues.
Acknowledgments
The authors acknowledge contributions made by Brad Mauger and Masaru Shiratori for cell culture consultation and data review; Neria Daniel for design of the VFD for agitator and heater temperature control; and the automation group and cell culture pilot plant for supporting pilot runs.
References
1 Diekmann S, et al. Single-Use Bioreactors for the Clinical Production of Monoclonal Antibodies: A Study to Analyze the Performance of a CHO Cell Line and the Quality of the Produced Monoclonal Antibody. BMC Proc. 5(sup 8) 2011; doi:10.1186/1753-6561-5-s8-p103.
2 Oosterhuis NMG, Van Den Berg HJ. How Multipurpose Is a Disposable Bioreactor? BioPharm Int. 24(3) 2011: 51–56.
3 Langer ES, Rader RA. Single-Use Technologies in Biopharmaceutical Manufacturing: A 10-Year Review of Trends and the Future. Eng. Life Sci. 14(3) 2014: 238–243: doi:10.1002/elsc.201300090.
4 Eibl R, et al. Cell and Tissue Reaction Engineering. Springer-Verlag: Berlin/Heidelberg, Germany, 2009: 55–82; doi:10.1007/978-3-540-68182-3.
5 Eibl R, et al. Disposable Bioreactors: The Current State-of-the-Art and Recommended Applications in Biotechnology. Appl. Microbiol. Biotechnol. 86(1) 2010: 41–49; doi:10.1007/s00253-009-2422-9.
6 Brecht R. Disposable Bioreactors: Maturation into Pharmaceutical Glycoprotein Manufacturing. Adv. Biochem. Eng./Biotechnol. 115, 2009: 1–31; doi:10.1007/10_2008_33.
7 Wood J, Mahajan E, Shiratori M. Strategy for Selecting Disposable Bags for Cell Culture Media Applications Based on a Root-Cause Investigation. Biotechnol. Progr. 29(6) 2013: 1535–1549; doi:10.1002/btpr.1802.
8 Hammond M, et al. Identification of a Leachable Compound Detrimental to Cell Growth in Single-Use Bioprocess Containers. PDA J. Pharm. Sci. Technol. 67(2) 2013: 123–134; doi:10.5731/pdajpst.2013.00905.
9 Xiao N, et al. Assessing the Risk of Leachables from Single-Use Bioprocess Containers Through Protein Quality Characterization. PDA J. Pharm. Sci. Technol. 20 June 2016; doi:10.5731/pdajpst.2015.006338.
10 Liu J. Profiling Leachables in Single-Use Biocontainers. PDA J. Pharm. Sci. Technol. 27 August 2015.
11 Horvath B, et al. A Generic Growth Test Method for Improving Quality Control of Disposables in Industrial Cell Culture. BioPharm Int. 26(6) 2013.
12 Mintz C. Single-Use, Disposable Products: A “State of the Industry” Update. Life Sci. Leader July 2009.
13 Kline S, et al. Change Notifications for Single Use Components: Criteria from an End-User Perspective. Pharm. Eng. 34(3) 2014.
14 Bellucci JJ, Hamaker KH. Evaluation of Oxygen Transfer Rates in Stirred-Tank Bioreactors for Clinical Manufacturing. Biotechnol. Progr. 27(2) 2011: 368–376; doi:10.1002/btpr.540.
15 Garcia-Ochoa F, Gomez E. Bioreactor Scale-Up and Oxygen Transfer Rate in Microbial Processes: An Overview. Biotechnol. Adv. 27(2) 2009: 153–176; doi:10.1016/j.biotechadv.2008.10.006.
16 Tribe LA, Briens CL, Margaritis A. Determination of the Volumetric Mass Transfer Coefficient (kLa) Using the Dynamic “Gas Out–Gas In” Method: Analysis of Errors Caused By Dissolved Oxygen Probes. Biotechnol. Bioeng. 46(4) 1995: 388–392; doi:10.1002/bit.260460412.
17 Urlaub G, Chasin LA. Isolation of Chinese Hamster Cell Mutants Deficient in Dihydrofolate Reductase Activity. Proc. Nat. Acad. Sci. 77(7) 1980: 4216–4220; doi:10.1073/pnas.77.7.4216.
18 Chen XN, et al. Charge-Based Analysis of Antibodies with Engineered Cysteines. MAbs 1(6) 2009: 563–571; doi:10.4161/mabs.1.6.10058.
19 Ma S. Analysis of Protein Therapeutics By Capillary Electrophoresis: Applications and Challenges. Dev. Biol. 122, 2005: 49–68.
20 Lazar AC, et al. Analysis of the Composition of Immunoconjugates Using Size-Exclusion Chromatography Coupled to Mass Spectrometry. Rapid Commun. Mass Spectrom. 19(13) 2005: 1806–1814; doi:10.1002/rcm.1987.
Kelsey Dent is research associate in protein analytical chemistry; Terry Hudson is director of SSFP drug-substance manufacturing sciences and technology; Edward Chan is technical specialist, and corresponding author Ekta Mahajan is principal engineer in pharma technical development operations and engineering at Genentech, Inc. (a member of the Roche Group), South San Francisco, CA; [email protected].
You May Also Like






