- Sponsored Content
- Gene Therapies
A Complete Solution for MSC Therapy Workflows: Cell Scale-Up, Cryopreservation, and DMSO RemovalA Complete Solution for MSC Therapy Workflows: Cell Scale-Up, Cryopreservation, and DMSO Removal
Sponsored by Corning

Corning HYPERStack 36-layer and 12-layer cell culture vessels
Mesenchymal stem cells (MSCs) are used frequently for cell therapy applications. As multipotent cells, they can differentiate into other lineages such as adipocytes, osteocytes, and chondrocytes. Additionally, they are known to secrete trophic factors that can play important roles in immunoregulation. Although MSCs can be isolated from several different tissue sources, those derived from bone marrow commonly are studied because they are easy to access in quantities large enough for therapeutic dosing (2 × 106 cells/kg of body weight). Still, that equates to 140 million cells for a 150-pound individual. And the process of expanding MSCs to achieve such quantities can introduce risks for heterogeneity-induced quality failures. Chances of clinical success can improve with a manufacturing process that maintains a homogeneous MSC population after expansion to meet required critical quality attributes (CQAs).
Once cells are scaled up, they need to be cryopreserved for stability and transport. Cryoprotectants such as dimethyl sulfoxide (DMSO) often are added to freezing media to reduce ice formation and increase cell survival after thawing. However, because DMSO can be cytotoxic, its final concentration in a drug product must be minimized. Tools from Corning Life Sciences can help scientists and drug developers meet growing demand for bone-marrow–derived MSC therapies.
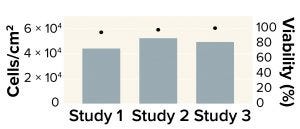
Figure 1: Expansion of human mesenchymal stem cells (MSCs) in a Corning HYPERStack-36 vessel; MSC densities ranging from 4.4 × 104 to 5.2 × 104 cells/cm2 were achieved after five days of culture. Across three studies, total MSC yield averaged 8.7 × 108 cells per HYPERStack-36 vessel, with >90% average MSC viability.
Mesenchymal Cell Scale-Up
MSCs are adherent cells that are sensitive to manufacturing process changes. That sensitivity can complicate scale-up to large quantities. When cultured under suboptimal conditions, MSCs can lose their multipotency. Corning HYPERStack 36-layer cell culture vessels offer a solution. A HYPERStack unit uses proprietary technology to provide a large surface area in a compact footprint. That technology relies on an ultrathin, gas-permeable film to facilitate gas exchange in each layer of the vessel. Each HYPERStack module comprises 12 individual chambers featuring Corning CellBIND surface treatment for optimal cell attachment. One module provides 6,000 cm2 of surface area; three modules can connect to form a HYPERStack 36-layer vessel, totaling 18,000 cm2 of growth surface area.
When human bone-marrow–derived MSCs are cultured in a HYPERStack 36-layer vessel, yields of >800 million viable cells can be achieved (Figure 1). Harvested cells show high viability and expression of markers demonstrating MSC multipotency (Figure 2). Such results show that large-scale expansion of MSCs in a HYPERStack vessel generates a homogeneous population of cells that maintain necessary CQAs.
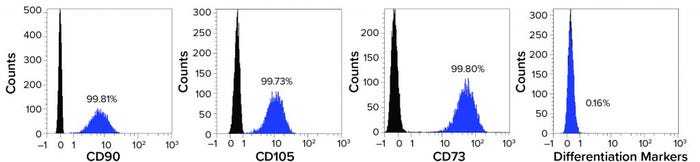
Figure 2: Mesenchymal stem cells (MSCs) recovered from a Corning HYPERStack 36-layer cell culture vessels show >99% expression of CD90, CD105, and CD73 markers while expressing <0.5% of differentiation markers (CD45, CD34, CD11b, CD19, and HLA-DR).
Corning cryopreservation bags remain flexible at ultralow temperatures
(e.g., –196 °C).
Large-Scale Cryopreservation
Cryopreservation of large quantities of cells has become an important strategy for simplifying cell therapy workflows because it increases product shelf life, allows time for quality testing, and lengthens the period of potential administration. Cryopreservation bags are designed for single-use storage, preservation, and transfer of large volumes of cells. Corning’s cryopreservation bags are novel bag-film containers that can remain flexible at ultralow temperatures (–196 °C) because they are made from a proprietary polyolefin–ethyl vinyl acetate blend. Corning produces the bags in four sizes covering fill volumes between 20 mL and 190 mL, with demonstrated performance for storage of bone-marrow–derived MSCs.
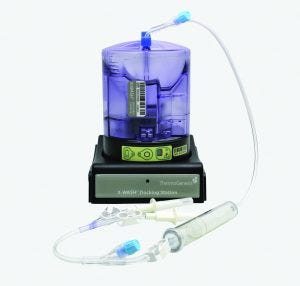
The Corning X-WASH system performs DMSO removal in a closed, sterile format.
DMSO Removal
DMSO can serve as a cryoprotectant for a wide range of cell types. It often accounts for 5–10% of a freezing solution to reduce ice formation and maintain cell viability. But because of its cytotoxic effects, DMSO must be removed as much as possible from a final cell therapy product. That can be accomplished by centrifugation and buffer exchange.
The Corning X-WASH system can perform those steps in sterile, closed conditions (Figure 3). The X-WASH system also uses highly sensitive infrared sensors and software to transfer process data from the X-WASH control module to a database. That feature supports good manufacturing practice (GMP) data processing, monitoring, and reporting. Ultimately, the X-WASH system is designed to wash, resuspend, and condense cell suspensions without compromising product quality.
Corning has used the X-WASH system to reduce the DMSO concentration from a bone-marrow–derived MSC product. About 70 million human MSCs were processed into Corning cryopreservation bags containing 10 mL of a 90% fetal bovine serum (FBS) and 10% DMSO solution. MSCs were thawed into 200 mL of phosphate-buffered saline containing 2% human serum albumin and 5% glucose. Cells were added to X-WASH cartridges for processing, then analyzed for recovery (Figure 4), viability (Figure 5), and multipotency (Figure 6). Ultraperformance liquid chromatography (UPLC) was used to quantify DMSO reduction. UPLC analysis showed that 200-mL dilution followed by a 200-mL wash in an X-WASH system reduced the final DMSO concentration by at least
40 fold.
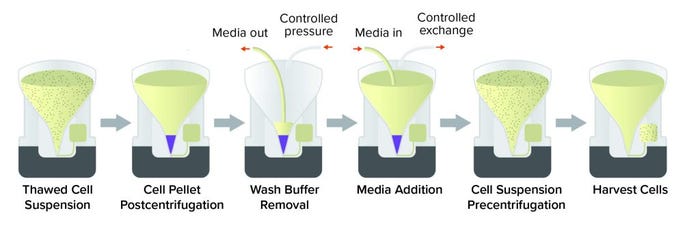
Figure 3: Corning X-WASH system workflow to remove dimethyl sulfoxide (DMSO)
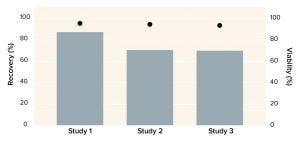
Figure 4: Recovery of human mesenchymal stem cells (MSCs) after washing with a Corning X-WASH system; the bars below represent MSC density, and cell viability levels are represented as dots.
Considerations for Closed Systems and Custom Media
Closed-system cell-culture products help reduce contamination risks during drug development and manufacturing. Thus, they should be considered when planning for cell-culture operations. Ordering multiple components and assembling tubing sets in house can add complexity and time to cell therapy processes. To aid in the development of such processes, Corning offers preassembled closed systems and aseptic-transfer caps that are compatible with many Corning cell culture vessels.
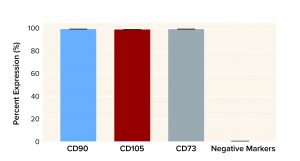
Figure 5: Human MSC multipotency as represented by average marker expression after processing with a Corning X-WASH system (with standard deviation, n = 3)
Corning closed-system solutions arrive at your facility sterile and ready to use. They mitigate contamination risks, reduce the time and expense of sourcing and assembly, and improve overall productivity. Moreover, Corning’s extensive library of fully validated filters, connectors, tubing, and clamps enables customized design of a closed-system solution for a specific application.
In cell-based therapies, cultured cells are the final product, requiring different manufacturing processes from those used for conventional biologics production. Major considerations in cell therapy scale-up include culture vessels and media as well as cells themselves. Inadequate attention to culture equipment and raw materials not only can diminish a therapy’s efficacy, but also can result in regulatory challenges that might delay a candidate’s progress through development. Because culture media are linked to cell growth and productivity, they rank among the most critical aspects of process development during scale-up.
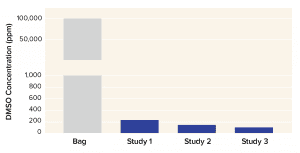
Figure 6: Dimethyl sulfoxide (DMSO) concentration in final product after 200-mL dilution followed by 200-mL wash in a Corning X-WASH system (data from three independent runs)
Although off-the-shelf media can provide fast and efficient solutions during early stages, they can have trouble meeting specific scale-up conditions later on. Moving from small-scale, small-volume, static cultures into large-scale, large-volume vessels can trigger a host of additional requirements that cannot be addressed easily using an off-the-shelf solution. Customization by a media manufacturer is an attractive solution to concerns associated with large production scales, including media stability, packaging, handling, and storage. Custom media solutions also help to derisk processing. Corning’s high-quality custom development and manufacturing services can produce tailored media and reagents to meet cell therapy production needs.
Simplifying Cell Therapy Workflows
Addressing the growing demand for cell-based therapies requires optimization of scale-up, cryopreservation, and DMSO removal. With some human MSC therapies requiring as many as one billion cells per dose, cell therapy companies need efficient ways to scale up production of homogeneous MSCs that meet CQAs. Additionally, large quantities of MSCs will need to be cryopreserved to simplify cell therapy workflows. Before product administration, DMSO and other reagents used during the manufacturing process will need to be reduced. Corning offers solutions to simplify the complete range of cell therapy workflows.
Hilary Sherman is senior scientist, and Chris Suarez is field applications manager at Corning Life Sciences, 836 North Street, Tewksbury, MA 01876; [email protected]; 1-800-492-1110.
CellBIND, HYPERStack, and X-WASH all are registered trademarks of Corning Incorporated.
You May Also Like





