- Sponsored Content
- Cell Therapies
- Facility Design/Engineering
A Plug-and-Produce GMP Plant for Cell and Gene Therapies Part 2: Rapid Deployment of a Commercial-Scale FacilityA Plug-and-Produce GMP Plant for Cell and Gene Therapies Part 2: Rapid Deployment of a Commercial-Scale Facility
Sponsored Content
 Extending the use of approved advanced-therapy medicinal products (ATMPs) to the tens of thousands of patients who could benefit from such treatment requires a 10- to 100-fold production scale-up. Given that each autologous ATMP batch yields one dose for one patient, expanding production throughput is not a question of boosting volume, but
Extending the use of approved advanced-therapy medicinal products (ATMPs) to the tens of thousands of patients who could benefit from such treatment requires a 10- to 100-fold production scale-up. Given that each autologous ATMP batch yields one dose for one patient, expanding production throughput is not a question of boosting volume, but  rather of amplifying single manufacturing runs. That is, scale-up is actually scale-out, and the dimensions of the ensuing endeavor extend beyond what occurs in the cleanroom.
rather of amplifying single manufacturing runs. That is, scale-up is actually scale-out, and the dimensions of the ensuing endeavor extend beyond what occurs in the cleanroom.
Coupled with each production run is a battery of controls for raw materials, in-process conditions, and product release. Higher throughput requires a smart facility layout that ensures segregated and controlled flows of materials, people, waste, and products. Increasing the number of processed batches by several times requires stringent process monitoring and validation based on expanded data pipelines.
In BPI’s September 2022 issue, part 1 of this report described a plug-and-produce (PnP) cell-manufacturing facility based on two core technologies: the ISO 13485–certified CliniMACS Prodigy mobile device and the patented ExyCell preconfigured and prefabricated facility modules. The integrated design is a ballroom-concept good manufacturing practice (GMP) “cell factory” for one to a dozen CliniMACS Prodigy instruments that enables fast-tracked construction, streamlined qualification, and modular expansion.
A pilot deployment in Shanghai’s ATLATL Innovation Center demonstrated the relatively short timeline and reduced cost of installing an integrated CliniMACS Cell Factory facility. Furthermore, this plant has confirmed that the PnP concept standardizes cell manufacturing processes, production environments, and supplies. Thus, high-quality GMP CliniMACS Cell Factory production units provide building blocks for scaling out ATMP production capabilities. Based on such a modular expansion strategy, routine production of 1,000 batches per year (about 22 batches per week), for example, would entail the installation of two ballrooms with 24 CliniMACS Prodigy instruments each and covering a combined surface area of about 200 m2.
Enhancements for Scale-Out
Evaluating the pilot GMP plant in the context of a commercial-scale facility highlights three areas for enhancement: manufacturing process modifications that completely integrate and automate the process from starting material to final filling; integration and automation of quality control and product release; and production facility adaptations that guarantee segregated and reproducible flows, secure traceability, and automated data handling (Table 1).
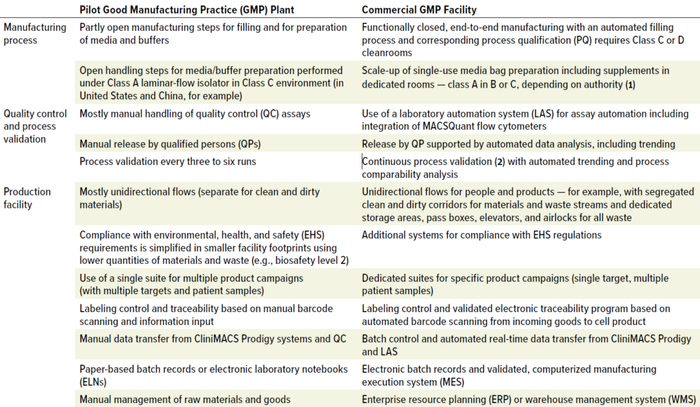
Table 1: Pilot-plant enhancements needed for commercial-scale advanced-therapy medicinal products (ATMPs) manufacturing.
The CliniMACS Prodigy platform is a closed, sterile system that automates every step of patient-specific cell manufacturing. Each system houses an end-to-end manufacturing process with controlled inputs and predefined outputs, which greatly simplifies the layout of a ballroom housing multiple CliniMACS Prodigy workstations, each station dedicated to a production campaign. At commercial scale, the end-to-end manufacturing process features media and buffer supply in single-use bags for each production batch. Stockpiling, movement, and use of those bags — along with all other materials entering and leaving the cleanroom — come through an enterprise resource planning or warehouse management system that collects barcoded information.
Automation-enabled devices and real-time data transfer for production monitoring, quality analytics, and product release are essential to commercial-scale manufacturing. Components of the necessary infrastructure include a laboratory automation system (LAS) with integrated MACSQuant flow cytometers for carrying out quality control (QC) assays from samples to results. Real-time data transfer from CliniMACS Prodigy instruments facilitates continuous process monitoring and electronic batch control. A computerized manufacturing execution system (MES) with electronic batch records monitors campaign-wide processing. The MES covers the complete manufacturing process from sample materials to formulated and finished final drug products.
ExyCell modules meet many layout requirements for commercial-scale production facilities. Approaches to airflow management, execution quality, and qualification remain the same as those for the pilot GMP plant. Adaptations address the needs of a larger facility that operates with nonexpert personnel (e.g., technicians)and processes greater quantities of materials and waste than the smaller pilot operation. The design secures segregated and reproducible flows of materials and waste while optimizing the arrangement and use of support areas around the core production environment. Appropriate systems ensure compliance with environmental, health, and safety regulations — e.g., fire protection, emergency exit routes, and waste disposal.
A Turn-KeyCommercial Facility
The CliniMACS Cell Factory platform complemented by ExyCell modules is a flexible, rapid-deployment solution to facilitate scale-out of ATMP production. With end-to-end closed processing, increasing throughput entails simply adding more CliniMACS Prodigy workstations to a campaign-dedicated suite. The reproducible layout and supply concepts simplify scale-out to a modular expansion in which integrated systems track materials flow, control manufacturing, and automate QC. Furthermore, planning experience and systematic documentation from suppliers provide for construction and qualification of a practically turn-key facility in months, ultimately shortening the time to market for promising autologous therapeutics.
References
1 PE 009-16. Annex 2A. Manufacture of Advanced Therapy Medicinal Products for Human Use. Guide to Good Manufacturing Practice for Medicinal Products. PIC/S Secretariat: Geneva, Switzerland, 1 February 2022; https://picscheme.org/docview/4590.
2 Ares1380025-30/03/2015. Annex 15: Qualification and Validation. EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use. European Commission: Brussels, Belgium, March 2015; https://health.ec.europa.eu/system/files/2016-11/2015-10_annex15_0.pdf.
Stefan Robert Kappeler is senior director of biopharma and regulatory at Exyte in Basel, Switzerland; Gordon Nicholson is director of biopharma and life science for northeast Asia at Exyte in Shanghai, China. Corresponding author Hermann Richard Bohnenkamp is vice president of business development for the APAC region ([email protected]); and Ulf Bethke is head of quality and regulatory affairs at Miltenyi Biotec in Bergisch-Gladbach, Germany. CliniMACS, CliniMACS Prodigy, and MACSQuant are registered trademarks of Miltenyi Biotec.
You May Also Like





