- Sponsored Content
- Facility Design/Engineering
A Plug-and-Produce GMP Plant for Cell and Gene Therapy — Part 1: Case Study in Modular Facility Design and DeploymentA Plug-and-Produce GMP Plant for Cell and Gene Therapy — Part 1: Case Study in Modular Facility Design and Deployment
Sponsored by Miltenyi Biotec
The use of approved advanced therapy medicinal products (ATMPs) remains limited despite their potential to address unmet medical needs. One example uses chimeric antigen receptor (CAR) T cells for treatment of refractory lymphoma (1). Typically, such medicinal products begin with cells that are harvested from a patient and genetically programmed to recognize and eliminate tumor cells upon reinfusion. Several cell therapies based on this and other technologies are approved for use in the United States, Europe, and China (2). Given their indications, up to 60,000 patients could be treated in those markets annually. However, in 2018 and 2019 only about 1,750 patients received CAR-T treatments, and eligible recipients faced long waiting lists with manufacturers (3). Autologous use of a patient’s own blood cells has significant consequences on the manufacturing and quality control (QC) of such therapies. In essence, one product for one patient represents one batch — and as a result, the methods established to produce conventional drugs cannot be transferred 1:1 to autologous ATMPs (Table 1).
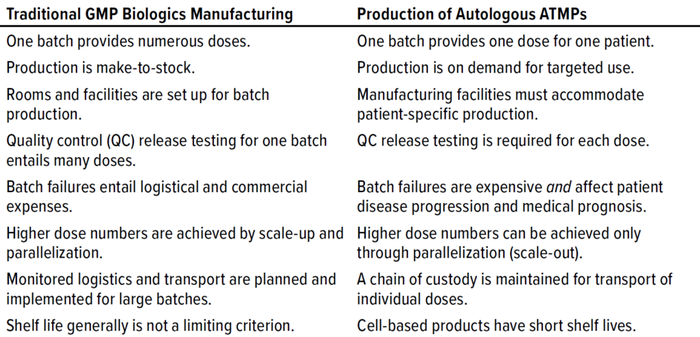
Table 1: Key differences between classical, batch-based pharmaceutical production and the production of autologous advanced therapy medicinal products (ATMPs).
Addressing the Bottleneck
Expanding the production of ATMPs to reach more patients poses numerous challenges (Table 2). Obstacles must be overcome at every stage of the manufacturing process, from handling the unique starting material of each batch to accommodating the high-throughput quality checks and complex delivery logistics for multiple batches. Autologous ATMPs are structurally complex. Any step in their manufacturing can compromise product quality, and process changes can adversely affect efficacy and safety.
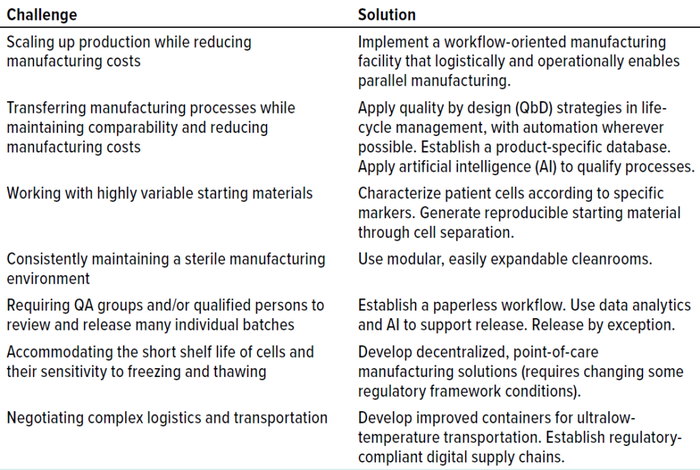
Table 2: Challenges and solutions for good manufacturing practice (GMP)–compliant manufacturing of advanced therapies; QA = quality assurance.
Ideally, all manufacturing and testing processes — from early development through commercial production — are scientifically understood and clearly defined. Good-practice (GxP) compliance is maintained throughout a product’s life cycle with controlled and easily validated process changes, production-scale changes, and technology transfers to other production sites. Also, carefully defining critical process parameters (CPPs) for production and release at an early stage — and keeping them constant over the entire life cycle of a product — greatly eases subsequent validation and qualification. Development of an ATMP should use the methodology of commercial production.
External conditions of production and testing should be determined simultaneously. By defining starting materials and raw materials in advance, critical material attributes and critical quality attributes (CQAs) can be established to fulfill requirements of the quality by design (QbD) methodology (4). Thus, process transfers from development to routine production and technology transfers to additional production sites can be easy and controlled. This approach shortens development times from R&D to clinic, thereby significantly reducing overall costs.
Here, we present a technical solution designed to keep parameters constant, from development to routine clinical production, and enable scale-out of autologous ATMP production through parallelization.
Plug-and-Produce Modular Manufacturing
The ISO 13485–certified CliniMACS Prodigy mobile device is used to manufacture ATMPs (5–8). It automates all steps in patient-specific cell manufacturing, including user-programmable sequences for magnetic cell selection, lentiviral vector transduction of cells, CO2-controlled cell expansion, temperature-controlled centrifugation, and conveyance of cells and liquids through sterile and fully enclosed tubing sets. The system is designed to introduce reagents and media safely, enable multiple applications using different tubing sets, drive high process reliability, and save users time and money.
Implementing a CliniMACS Prodigy system for manufacturing requires a dedicated production facility. With modular design and integrated elements for air conditioning, lighting, fire protection, power and data cables, sensors, and supply lines, ExyCell modules simplify construction (9). A plenum-integrated filter-fan unit (PIFF) for ceiling air return and a plenum-attenuated filter-fan unit (PAFF) for low-level air return help a facility meet regionally different good manufacturing practice (GMP) requirements. Bundled utility tapping points supply process equipment with power, air, and water. The modular system balances standardization with design freedom to adapt each process environment to developed production processes and optimal operations. An ExyCell module can be installed in an existing building, or a cost-effective prefabricated building can be created as the outer shell.
Those complementary solutions have been integrated into a standard layout for a CAR-T production facility that provides GMP-compliant capabilities and is adaptable to user needs with minimal planning required. The overall goal is to facilitate transferring CAR-T and related cell-engineering methods developed at universities and specialized start-up companies to a current GMP (CGMP)–compliant production environment. Manufacturing capacities are made available quickly by keeping basic requirements for setting up the production environment standard.
The layout is modularly expandable and includes a central production environment. Support areas (e.g., warehouses, laboratories) are built adjacent to cleanrooms using standard building materials (Figure 1). Because production is functionally closed, multiple individual batches can be produced in parallel in a central cleanroom, which optimizes operating processes and helps reduce product costs. To ensure flexibility and future viability, the plant is automated as a modular “plug-and-produce” (PnP) system (10).
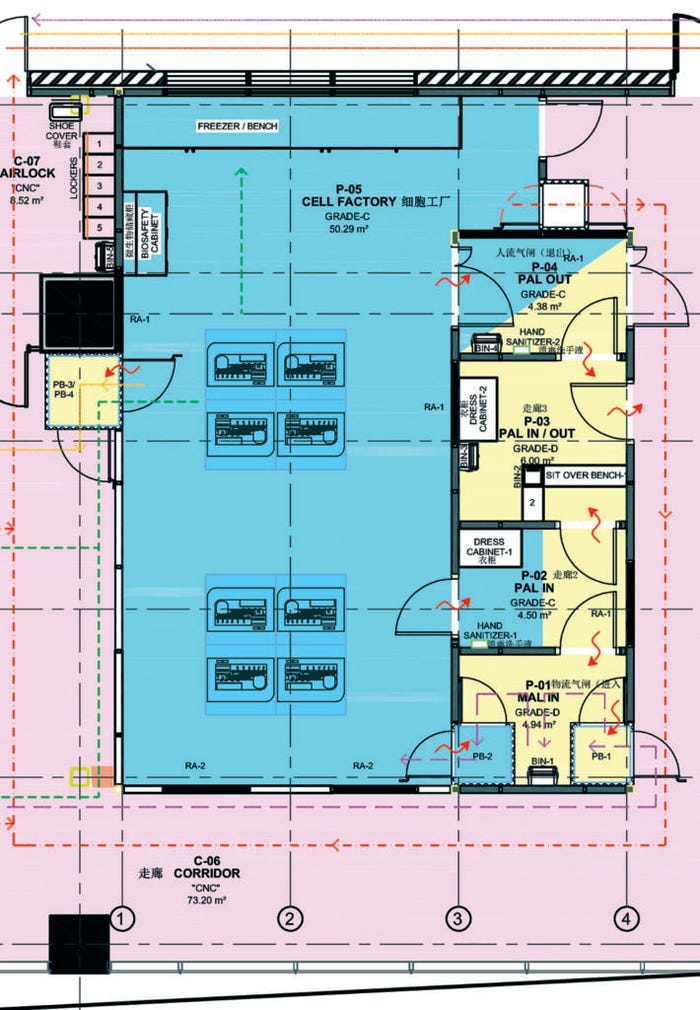
Figure 1A: Layout of the CliniMACS cell factory in Shanghai, China; blue, yellow, and pink indicate cleanroom grades C, D, and CNC (controlled, nonclassified), respectively.
The facility has an optimized workstation layout, with two sets of four stations and the ability to add a third set later. This layout is oriented for efficient flow and high productivity. As a biosafety level 2 cleanroom, it makes personnel and material flows unidirectional, with a sequential gowning concept from controlled, nonclassified (CNC) space to grade-C areas. All materials are brought in through active pass boxes, and all waste is sterilized through a two-door decontamination autoclave.
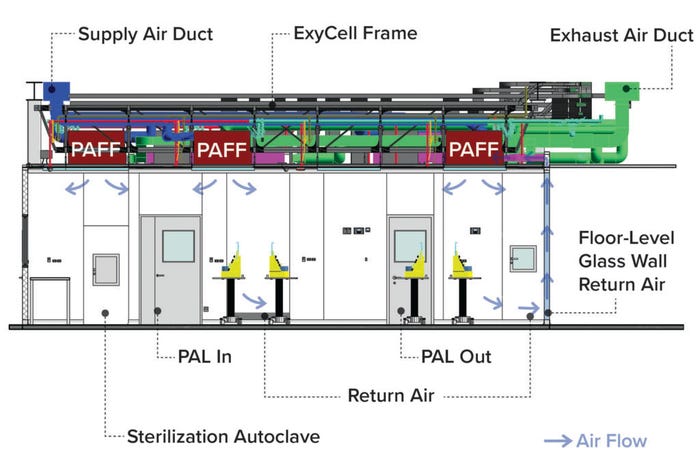
Figure 1B: Side view of the CliniMACS cell factory in Shanghai, China; PAFF = plenumattenuated filter-fan unit, PAL = personnel airlock.
A central supply station feeds individual workstations from the ceiling, which facilitates later adjustments. Ceiling connections can be relocated with minimal impact on operations, and additional utilities can be added when needed. Final connections and terminations are cut into the ceiling; major adjustments are made by replacing a single 1.2 × 1.2 m ceiling tile using existing penetrations and prewired connections. The design also includes required slopes for clean media lines across the entire cleanroom area.
With this GMP plant concept, project-specific technical information such as bills of materials, prices, and schedules can be generated directly even in the early design phase. That greatly reduces commercial, scheduling, and quality risks in planning and execution. However, adjustments can be made up to the implementation phase and later, with minimal effort and impact.
A Prefabricated Production Facility in Shanghai
To test the feasibility of the concept and examine options based on a proof of concept, a facility for clinical sample production was built in Shanghai’s ATLATL Innovation Center based on the layout described herein (12). This facility complies with CGMP regulations; meets numerous local regulations and standards for buildings, installations, and occupational health and safety; and integrates the necessary functions to produce and release ATMPs from a small footprint.
The project-specific design process took two months from concept initiation to documents submission and regulatory approval. That included a redesign to create a lightweight structure version for retrofit into an existing building, a task that was simplified by the overall robustness of the concept. Designed modules were prefabricated in two weeks, tested, and packed for transport along with most of their intermediate connections (including those for clean utilities) and the corresponding commissioning and qualification documents. Modules were delivered to the construction site by conventional flat-pack transport. The PnP approach allowed for easy integration with the preconfigured infrastructure. Onsite, most connections had only to be attached to prepared supply systems, saving significant time (10). Heating, ventilation, and air conditioning (HVAC), process utilities, and other supporting infrastructure were provided right next to the modular plant in this project.
Site preparation and installation of support structures began in the middle of December 2020, after the order for the modules was confirmed early that month. Modules were delivered to the site on December 26. The Chinese New Year in early February and pandemic-related delays extended the overall schedule to the end of March 2021, mostly because of lead times for delivery of wall systems and for design and installation of a dedicated qualified building management system (Q-BMS). Utilities connections were timed to prevent interference with ongoing R&D operations elsewhere in the building.
After the modules and supply lines were installed, the walls were attached to the grids of the modular ceiling, and a vinyl floor was laid. Unlike a conventional modular system, the module connections are invisibly integrated into the design (Figure 2). Structurally, the modules are supported by their own frames; they also can be suspended from an existing building structure at heights that are fully adjustable to meet process-specific requirements. Additional modules can be added at any time without interrupting ongoing production in existing modules. The onsite plant-engineering team can add or remove walls and doors as well as adjust adjacent filter-fan units.
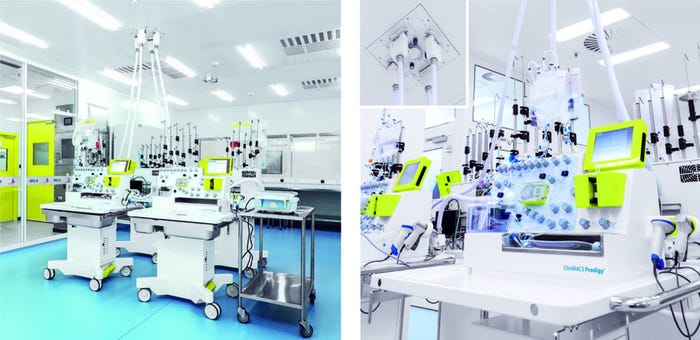
Figure 2: Finalized CliniMACS cell factory in Shanghai, China (left) with a workstation of four CliniMACS Prodigy instruments fed from a central supply in the ceiling (right).
Several modifications were made before commissioning and qualification in April: integration of fully cleanable floor-level return air-duct systems into the glass walls, addition of PnP sockets, and installation of fully glazed perimeter walls with 90-minute fire resistance. Plant and cleanroom qualification of GMP-critical design elements were performed in all systems with direct influence, according to defined qualification plans adapted to the plant and incorporating factory acceptance test (FAT) results where possible (11). No critical deviations were found.
It is worth noting that Chinese cleanroom regulations changed at the end of 2021 to require floor air return in all plants to be classed as B or C. Thus, the original module design using PIFFs for ceiling air recirculation was adapted with a combination of recessed return-air shafts to the ceiling and a unique glass return-air shaft wherever walls are full-height glass. The floor-to-ceiling glass is advantageous in this kind of facility. Such walls are easy to clean, meet all CGMP criteria, and allow inspectors, visitors, and supervisors to view operational areas without gowning up or disrupting ongoing operations. Transparency also serves communication and rapid assistance in the event of an incident. Finally, high visibility encourages an organized and tidy work environment, which reduces the risk of contamination, and work quality increases when there is a line of sight to other employees and the outside world.
Paving a Path to Commercialization
Most cell and gene therapy start-ups lease space to convert into a facility for small-scale ATMP production, with the intent to build owned commercial-scale facilities after a successful market launch. The lightweight design of this PnP modular production concept is ideal for such purposes because of fast assembly, easy delivery to an existing building, and a clearance height of at least 2.7 m in a space that is only 4 m high. All maintenance can be performed from the front in a mechanical room, from inside cleanrooms, or from above.
A building load of just 80 kg/m2 allows the CliniMACS Prodigy system to be installed into most buildings with minimal or no support structure. It can be supported from the floor (as in this project) or suspended from the building superstructure. Thus, this integrated solution coordinates, optimizes, and standardizes a central manufacturing process and production environment, including cleanrooms and their supplies. The modular PnP concept reduces timelines and costs of implementation while accommodating future scale-out and expansion of a GMP plant.
Looking Ahead
Implementing the pilot plant described above provided an opportunity to examine the feasibility of creating high-quality integrated cleanroom facilities for autologous ATMP production. The experience established a path to regulatory approval, tested the project sequence and supply chain, and revealed areas to optimize. Plans for improvements include shortened project timelines and innovative elements that will facilitate predictive maintenance, enable easy wall installation or removal with requalification within three days, and integrate industrial internet of things (IIoT) concepts for plant and process control. Next month, Part 2 will conclude this report by discussing the use of this PnP concept for commercial-scale manufacturing facilities.
References
1 Sehn LH, Salles G. Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 384(9) 2021: 842–858; https://doi.org/10.1056/nejmra2027612.
2 Cao X, et al. China Enters CAR-T Cell Therapy Era. Innovation 3(1) 2021: 100197 https://doi.org/10.1016/j.xinn.2021.100197.
3 Bennett C. CAR T-Cell Therapies: Early Insights into Access and Affordability. ObR Oncol. 11(6) 2018; https://obroncology.com/article/car-t-cell-therapies-early-insights-into-access-and-affordability.
4 ICH Q8(R2). Pharmaceutical Development. US Fed. Reg. 71(98) 2009; https://database.ich.org/sites/default/files/Q8_R2_Guideline.pdf.
5 Apel M, et al. Integrated Clinical Scale Manufacturing System for Cellular Products Derived By Magnetic Cell Separation, Centrifugation and Cell Culture. Chem. Ing. Tech. 85(1–2) 2013: 103–110; https://doi.org/10.1002/cite.201200175.
6 Kaiser AD, et al. Towards a Commercial Process for the Manufacture of Genetically Modified T Cells for Therapy. Cancer Gene Ther. 22(2) 2015: 72–78; https://doi.org/10.1038/cgt.2014.78.
7 Mock U, et al. Automated Manufacturing of Chimeric Antigen Receptor T Cells for Adoptive Immunotherapy Using CliniMACS Prodigy. Cytother. 18(8) 2016: 1002–1011; https://doi.org/10.1016/j.jcyt.2016.05.009.
8 Jackson Z, et al. Automated Manufacture of Autologous CD19 CAR-T Cells for Treatment of Non-Hodgkin Lymphoma. Front. Immunol. 7(11) 2020: 1941; https://doi.org/10.3389/fimmu.2020.01941.
9 Mussati L, et al. Ceiling Module for the Construction of a Clean Room. US Patent Application 16/512,451, filed 21 January 2021.
10 Jebari A, et al. Planung und Realisierung von Modularen Plug und Produce-Systemen. TechnoPharm 11, 2021: 72–78.
11 Pharmaceutical Engineering Guide for New and Renovated Facilities: Commissioning and Qualification, Volume 5, Second Edition. ISPE: North Bethesda, MD, June 2019; https://ispe.org/publications/guidance-documents/baseline-guide-vol-5-commissioning-qualification-2nd-edition.
12 Kappeler SR, et al. Realisierung einer Standardisierten GMP Anlage zur Herstellung von Personalisierter Medizin. TechnoPharm 11, 2021: 184–193; https://www.ecv.de/beitrag/TechnoPharm/Realisierung_einer_standardisierten_GMP Anlage_zur_Herstellung_von_personalisierter_Medizin.
Stefan Robert Kappeler is senior director of biopharma and regulatory at Exyte in Basel, Switzerland; Gordon Nicholson is director of biopharma and life science for northeast Asia at Exyte in Shanghai, China. Corresponding author Hermann Richard Bohnenkamp is vice president of business development for the APAC region ([email protected]); and Ulf Bethke is head of quality and regulatory affairs at Miltenyi Biotec in Bergisch-Gladbach, Germany. CliniMACS and CliniMACS Prodigy are registered trademarks of Miltenyi Biotec.
The CliniMACS® Cell Factory enables parallel cell manufacturing of patient-derived cell products on multiple instruments – in one room. Get more info here.
You May Also Like





