- Sponsored Content
- Gene Therapies
- Single Use
Advancing Logistics for ATMP ManufacturingAdvancing Logistics for ATMP Manufacturing
April 19, 2022
Sponsored by Single Use Support
Advanced therapy medicinal products (ATMPs) hold much potential for improving healthcare. They offer hope for treating or even curing patients. The biopharmaceutical industry has recognized the importance of making such therapies accessible to as many people as possible. To provide personalized ATMPs, biomanufacturers are shifting toward flexible, patient-centered production processes.
A Paradigm Shift in ATMP Manufacturing
Ex vivo cell and gene therapies are particularly promising approaches to personalized regenerative medicine. Thus, it is no surprise that the numbers of US Food and Drug Administration (FDA) approvals for cell and gene therapies are rising for a broader swath of indications. By implication, the number of eligible patients is increasing. Significant interest in ATMPs from both patients and biotechnology companies is driving the biopharmaceutical industry to create an infrastructure for sustainable supply of raw materials and process components used in ATMP manufacturing.
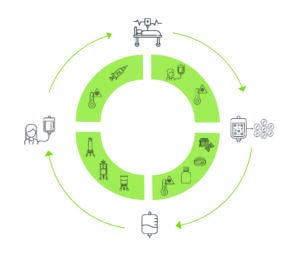
Figure 1: A typical manufacturing process for an autologous cell therapy, comprising multiple steps for collection of patient cells, upstream production, downstream processing, and clinical application.
An autologous cell manufacturing process begins with collection of samples from a patient, typically through apheresis or biopsy of bone marrow or tumor tissue (Figure 1). During upstream production, source cells undergo gene-editing steps that add, replace, edit, and/or silence genes with the goal of enhancing the resulting product. After gene modification and activation, cells are cultured in growth media, where they proliferate for several days, increasing their viable cell densities. Finally, cultured cells are harvested and cryopreserved.
Downstream processing of harvested biological material can be difficult at small scales. Chromatography, ultrafiltration/diafiltration (UF/DF), and sterile filtration are performed to remove unwanted impurities, reagents, and/or particles and to reduce the volume of a drug substance. After quality and release testing, a finished drug product is transported back to a patient for clinical administration.
Inherent complexity arises with the need to perform many process steps, some of which can occur in different venues. Critical process steps along the cold-chain logistics make the process even more difficult. Cells must be cryopreserved after most process stages and before transport to help ensure optimal product quality. Precise, sterile fluid transfers and product protections are needed at every stage to preserve product integrity and sterility during aliquoting. Robust protective solutions can reduce product loss and minimize contamination, and logistics must be aligned between hospitals and manufacturers to safeguard the cold chain.
Building a comprehensive logistical structure for ATMPs supports the biopharmaceutical industry’s goal of helping patients to experience the best possible outcomes. It is critical to have reliable consumables and platforms for drug-product homogenization, freeze–thaw, and fill–drain. Speed and safety during operation also are of utmost importance in cold-chain logistics to optimize drug products’ potential to treat patients.
Driving Automation and Bioprocessing 4.0
There is great potential in automating process steps to address complexities in ATMP production logistics and to facilitate implementation of manufacturing 4.0 principles.
Many batch failures in manufacturing facilities derive from human errors. Having a safe and automated process in place enables both archiving of relevant data and improvement of a manufacturing environment’s status quo. Product quality can be increased through standardized procedures. Costs also can be minimized through reduced loss rates and working hours.
Technologies that can be advanced through automation include those used in fill–drain, freeze–thaw, and product-homogenization steps. Operators sometimes need to massage single-use bags for hours to regain product homogeneity. But product temperature rises uncontrollably during such contact. Therefore, the level of homogenization can vary from bag to bag depending on handling intensity.
But new opportunities for standardization come with automation. The transition also brings biotechnology companies closer to bioprocessing 4.0: Only automated processes can be digitalized and driven by artificial intelligence (AI).
Each process step can yield performance data that can be collected to optimize ATMP manufacturing. Data can be reported either to a company’s manufacturing execution system (MES) or to radio-frequency identification (RFID) tags. The latter can be attached to single-use systems (e.g., two- or three-dimensional bioprocess containers) or to single-use assemblies with the goal of introducing sterile consumables into the internet of things (IoT). The RFID tags then would trigger communication between devices responsible for monitoring products and processes.
For example, a single-use bag filled with a drug product now can be equipped to correspond wirelessly with platform systems such as automated fill–drain or freeze–thaw units. To optimize product quality or to streamline process efficiency, it is possible to automate recipe choice, performance speed, and other relevant parameters. Smart manufacturing systems now can collect data, identify relevant parameters, and control processes automatically.
Best Practices for Viral Vector Manufacturing
Flexible and modular systems are the way forward to meet requirements for ATMP scalability. Recipes that transfer from small to large scales, processes that perform the same way across different scales, and speed to market all are critical needs.
ATMP manufacturing is not limited to small batch sizes. Cells derived from single patients are processed at such scales, but lentiviral (LV) vectors, for example, are made in large batches. Thus, viral vectors can help to accelerate the development of gene and gene-modified cell therapies.
Batch volumes for LV vectors can range from below 1 L to 100 L and beyond while maintaining the same size of single-use bag. Again, automation can help companies cope with increased batch sizes and handle large numbers of single-use bioprocess containers. It also can make drug-product dispensing and sealing safer and more efficient than they would be with manual processing.

Figure 2: Filling of viral vectors into small bioprocess containers using a RoSS.FILL CGT system (Single Use Support).
Innovations in the single-use industry are facilitating implementation of automated, good manufacturing practice (GMP)-compliant platform systems that can perform quickly and accurately. For example, it is now possible to integrate automated sealing valves into filling platforms to enable hands-free sealing of primary packaging from single-use assemblies (Figure 2). The delicate process step of securing sterility during sealing now can be eliminated. Pioneering biopharmaceutical suppliers also have found room for improvement in many other ATMP manufacturing processes.
The Bright Future of ATMP Manufacturing
Cell and gene therapies are already an important part of the biopharmaceutical industry and are becoming a stable part of the market. Their progress will revolutionize manufacturing and distribution of pharmaceutical drug products. Current limitations also provide the impetus for companies to break free from rigid manufacturing processes, dependencies on select suppliers, and inflexible single-use technologies.
Seamless scale-up of ATMP or vaccine production requires flexible — hence modular — platforms. Establishing a rich supply chain of single-use systems that can be made available with short lead times will pave the way for implementing such technologies in ATMP manufacturing logistics.
One way to accomplish that lies in developing strong partnerships between established industry players in the (bio)pharmaceutical industry and innovator organizations that supply them with novel methodologies, fresh perspective, and innovative components that can facilitate the adoption of bioprocessing 4.0 principles. With early adoption comes greater preparedness for change.
Daniel Tischler is team lead for the Platform Engineering and Innovation group at Single Use Support GmbH, Endach 36, 6330 Kufstein, Austria; [email protected]; https://www.susupport.com.
You May Also Like





