- Sponsored Content
- Gene Therapies
Allogeneic Cell Therapy Manufacturing: Preparing for Tomorrow’s SuccessAllogeneic Cell Therapy Manufacturing: Preparing for Tomorrow’s Success
December 6, 2022
Sponsored by Cellistic
Cell therapies are promising new drug products that treat or cure diseases that, until the past decade, had no other treatment options. Several autologous cell therapies have been approved, and their efficacy has been proven, especially in immunooncology. However, autologous therapies can present some difficulties for both developers and patients (e.g., short timelines, point-of-care drug administration). Allogeneic cell therapies are not associated with those challenges. For example, patient access to an autologous treatment can take months, time that patients with fast-progressing diseases might not have.
Having an allogeneic cell therapy that is ready to use immediately is advantageous. Starting materials for autologous cell therapies often are suboptimal because of immune-cell exhaustion and the health of the patients from which those materials are sourced. Cells manufactured for allogeneic therapies can be sourced from healthy donors and be fully characterized. Thus, the risk of patient-to-patient variability can be mitigated.
Another benefit developing allogeneic cell therapies is cost. Generating an allogeneic product in large batches to serve multiple patients leads to a more standardized and predictable process compared with the autologous process that serves one or a few patients. The allogeneic process has a higher success rate, and biomanufacturers can take advantage of better economies of scale, thereby reducing cost per patient.
For those reasons, the number of allogeneic cell therapies in development is growing. Since 2017, the number of allogeneic cell therapies starting preclinical and clinical trials has increased at an average of 12.5% each year (1). One market research study estimates that the allogeneic cell therapy market is expected to grow at a compound annual growth rate (CAGR) of 9.6% from 2022 to 2027 (2).
Although biopharmaceutical developers increasingly are interested in allogeneic cell therapies, the industry is not leaving autologous therapies behind. In a survey of >500 biopharmaceutical employees, about half of the respondents were neutral on the question of moving away from autologous cell therapies, in both big biopharmaceutical companies and start-ups (Figure 1).
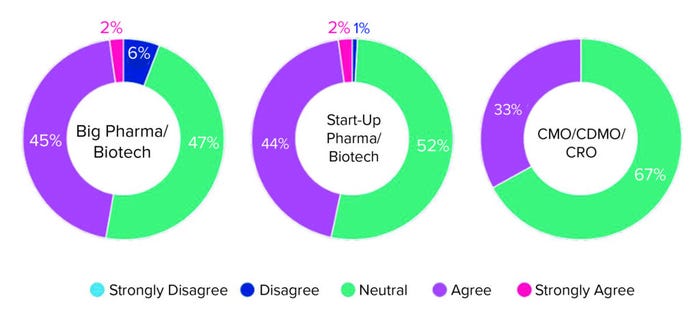
Figure 1: Survey responses to the question “Do you anticipate your company moving
away from autologous cell therapies in the next five years?”
One explanation for that reluctance is that most companies are hesitating to engage until long-term efficacy data for allogeneic therapies are available to justify the investment in what is still an emerging modality (3). Another reason to keep interest in autologous therapies is the complicated process of building a strategy for scale-up to commercial volumes for allogeneic cell therapies. Despite the progress made over the past decade, biomanufacturers continue to have difficulties in expanding allogeneic cultures with minimal risk of limiting therapy efficacy, safety, and efficiency. Below, we describe the key factors to take into consideration when establishing a process for allogeneic cell therapies.
Cell Sourcing and Reprogramming
Donor sourcing and selection is one of the earliest and biggest challenges in upstream cell therapy development. That is because disease targets require highly specific donor attributes (including age, disease state, and absence of adventitious agents). Companies can have difficulty finding donors who have the desired and possibly rare characteristics needed and to obtain the informed consent needed as a therapy progresses from the R&D stages to manufacturing for clinical trials, and eventually for market approval.
Donor cells can be either mature cells that can be expanded or cells in a multipotent state. Mature cells are subject to variability (e.g., because of genetic differences and health conditions), that should be monitored continuously. That variability risk can be reduced by using multi- or pluripotent cells such induced pluripotent stem cells (iPSCs).
iPSCs are obtained from adult donors’ somatic cells. The cells are reprogrammed to pluripotency using a combination of genetic elements delivered into the cells by viral or other means. Reprogramming should be performed using a method that is not only efficient in generating iPSCs, but also safe in terms of not imposing a risk of insertional mutagenesis, karyotype instability, and residual transgene expression. The prominently used reprogramming methods include the use of sendai viral vectors, episomal vectors, and mRNA transgenes. Other technologies also are being developed.
iPSCs proliferate indefinitely and maintain the potential to differentiate to nearly any functional cell type in the body. Thus, the cells can be gene edited, expanded, and characterized, to create master cell banks that can be used for every product batch. Because of those attributes, iPSCs have become one of the preferred starting materials for cell therapy development. One study has projected iPSC use to grow to 12% of all cell therapies in development by 2032 (4).
Gene Editing
The greatest advantage of manufacturing iPSC-based allogeneic cell therapies is accessibility to an unlimited cell source that is readily amenable to genetic modification. iPSCs can undergo multiple genetic editing rounds to make starting material histocompatible to multiple donors and alleviate the risk of graft-versus-host-disease and graft rejection by a host.
Gene modification can improve cell therapy efficacy. Developers can equip cells with “safety switches” and performance enhancers. But the more genes are edited, the higher the risk of safety issues such as off-target effects. Manipulations requiring clonal expansion of iPSCs (e.g., reprogramming and genome editing) can lead to karyotype abnormalities if such manipulations are not carefully performed. A well-planned gene editing design process is key to mitigating such risks. Considerations for such a process include exhaustive genotype screening and thorough characterization of master cell banks and final products.
Process Development and Scalability
Scalability is important for allogeneic cell therapy development, but it can be difficult to achieve. The goal is to build a controlled manufacturing process that reliably can reproduce the cell yield and quality generated in early development stages for clinical trials and commercial scales. Starting materials usually undergo an expansion process and then are transferred into large-scale platforms such as bioreactors, which can be used sequentially to generate the cell quantities required for allogeneic cell therapies (5).
The above strategy must be taken early to develop an effective development process and to ensure availability of the required expertise and equipment to achieve good manufacturing practice (GMP)-compliant scaled manufacturing. Communicate with manufacturing experts and developers of devices and media to avoid potential bottlenecks and enable robust commercial-scale manufacturing.
Working with a Manufacturing Partner
Allogeneic cell therapies can treat millions of patients cost efficiently and effectively. Biopharmaceutical companies face multiple challenges in cell therapy development, and they need to think strategically about manufacturing early in the process. Producing predictably differentiated, characterized, and functional cells at large scale also requires the kind of development and production expertise that comes from years of experience.
An experienced manufacturing partner can offer the required knowledge and expertise in process development and manufacturing. A partner company should know how to handle cells; how to verify cell type, potency, and functionality; and how to manufacture safe and efficacious end-products at scale. Working with the right partner should result in a development and manufacturing strategy that will bring the best treatments to patients.
References
1 Cao M. Manufacturing Challenges and Considerations for Allogeneic Cell Therapy. Hanson Wade: 2022; https://beacon-intelligence.com/infographic/manufacturing-challenges-considerations-for-allogeneic-cell-therapy.
2 Allogeneic Cell Therapy Market Report. Global Market Estimates: Altadena, CA, 2021; https://www.globalmarketestimates.com/market-report/allogeneic-cell-therapy-market-3894.
3 Walters P. Horizons: Life Sciences. CRB Group 2021; https://go.crbgroup.com/horizons-life-sciences-report.
4 Cellistic Strategy Input. Triangle Insights Group: Durham, NC, 2022.
5 Lee B, et al. Cell Culture Process Scale-Up Challenges for Commercial-Scale Manufacturing of Allogeneic Pluripotent Stem Cell Products. Bioeng. 9(3) 2022: 92; https://doi.org/10.3390/bioengineering9030092.
Elena Matsa is vice president of cell technology at Cellistic, rue Adrienne Bolland 47, 6041 Gosselies, Belgium; [email protected]; https://www.cellistic.com.
You May Also Like





