- Sponsored Content
- Gene Therapies
Autologous CAR T Cell Manufacturing Using a Semiautomatic, Closed, Modular Workflow: Seamless Transition from Discovery to Clinical ManufacturingAutologous CAR T Cell Manufacturing Using a Semiautomatic, Closed, Modular Workflow: Seamless Transition from Discovery to Clinical Manufacturing
August 27, 2021
Sponsored by Thermo Fisher Scientific
Chimeric antigen receptor (CAR) T cell therapies have advanced rapidly in recent years, with a number of targets in clinical research and several US Food and Drug Administration (FDA)-approved products already on the market. There has been tremendous effort to make CAR T cells more effective, safe, and persistent when treating patients. On the manufacturing side, however, errors, lot-to-lot variation, and contamination can be associated with open processes and manual handling of CAR T cells.
Cell isolation, gene editing, expansion, and cryopreservation are complex steps in a typical autologous CAR T cell manufacturing process. Integrating this complicated multistep workflow into a closed, modular, benchtop system can facilitate transitions from laboratory scale to clinical manufacturing and improve a CAR T cell product’s consistency, purity, and safety. Thermo Fisher Scientific presents a digitally compatible, good manufacturing practice (GMP)-compliant, semiautomated manufacturing platform, which when used with Gibco™ CTS™ reagents, protocols, and analytics can result in consistent, efficacious CAR T cell production.
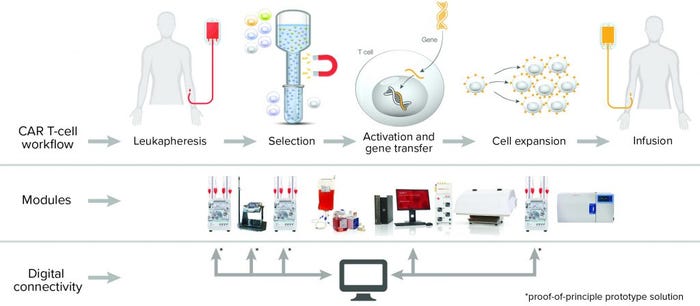
Figure 1: (top) CAR T cell therapy workflow; (bottom) workflow solutions from Thermo Fisher Scientific
Fill out the form below to read the complete white paper now.
You May Also Like





