- Sponsored Content
- Bioreactors
- PAT
- Process Development
A Rapid, Low-Risk Approach Process Transfer of Biologics from Development to Manufacturing ScaleA Rapid, Low-Risk Approach Process Transfer of Biologics from Development to Manufacturing Scale
Sponsored by Sartorius
 Successful scale-up of cell culture for manufacturing of biopharmaceuticals gives companies time to accelerate clinical development, product commercialization, and market access (1). Scaling a cell culture process in stirred-tank bioreactors ideally includes optimizing that process at laboratory scale and then transferring it through larger pilot-scale and finally to manufacturing-scale bioreactors (2). This is a complex, time-consuming business that can involve process transfer — sometimes to different geographical locations and through many sizes of bioreactors, each of which can operate according to different agitation principles and gassing strategies (3). Therefore, each scale might require several runs to obtain similar results, and optimization also might be required at the different scales.
Successful scale-up of cell culture for manufacturing of biopharmaceuticals gives companies time to accelerate clinical development, product commercialization, and market access (1). Scaling a cell culture process in stirred-tank bioreactors ideally includes optimizing that process at laboratory scale and then transferring it through larger pilot-scale and finally to manufacturing-scale bioreactors (2). This is a complex, time-consuming business that can involve process transfer — sometimes to different geographical locations and through many sizes of bioreactors, each of which can operate according to different agitation principles and gassing strategies (3). Therefore, each scale might require several runs to obtain similar results, and optimization also might be required at the different scales.
Scale-up challenges occur when bioreactors used during process transfer differ significantly in geometry and design. Different mixing methods, impeller types, and sparging strategies all can compromise cell-culture performance (3).
To ensure a quality by design (QbD) approach during cell-culture scale-up, companies use scaling strategies such as volumetric gas-flow rate (VVM), power per unit volume (P/V), and mass transfer coefficient (kLa) to determine scale-dependent parameters including gas-transfer rates and agitation rates in larger bioreactors (4) and scale-down models (SDMs) (5, 6).
The established method of scaling up a bioprocess is based on keeping a single parameter constant throughout the complete range of scales. That can cause problems when developing a process across a broad range of scales from process development (starting at 15 mL) to production (2,000 L). During scale-up, key process indicators (KPIs) including viable cell concentration (VCC), cell viability, cell diameter, and product titer all can be affected (7). Also, process transfer can influence a biologic’s critical quality attributes (CQAs) such as its glycan profile (7) because scale-dependent and/or -independent parameters including temperature and pH have not been optimized using design of experiments (DoE) principles to develop a robust process before transfer to another scale.
One approach for overcoming several scale-up issues is to use miniature bioreactors for process development. They offer geometrical height-to-diameter (H/D) ratios similar to those of larger vessels. A process can be transferred across a range of manufacturing-scale bioreactors with similar geometries (8). Having detailed knowledge of vessels that will be used throughout a scale-up process allows upfront consideration of their limitations during process development and before scale-up. That enables calculations to be made — of process parameters such as agitation rate —according to the physical capabilities of the vessels used. For example, a specific power input of 30 W/m³ represents a working stirrer speed at the 2,000-L scale, which often leads to nonoptimal conditions such as low stirring speeds in minibioreactors. Finally, scalable analytical tools need to be used alongside the bioreactors to ensure that a process can be monitored and measured in the same way throughout scale-up. That allows for development of a robust process because on-/in-line measurements (with feedback loops) do not depend on users performing measurements off-line. The process then can be transferred through different scales, often with fewer scale-up steps, to speed up a cost-effective process transfer and enable use of smaller-footprint facilities.
We used ambr bioreactors (Sartorius) as the small-scale bioreactors in this study because they can be run in a high-throughput, multiparallel way with a completely automated system, which is perfect for DoE experiments. Such bioreactors are replacing glass laboratory-scale vessels, with over 80% of the biopharmaceutical industry now using them as mimics of benchtop bioreactors for process development in fed-batch and batch cell culture (9).
Real-time data on cell-culture parameters including dissolved oxygen (DO) and pH values are logged continuously by the ambr cell-culture workstation. Cell culture KPIs such as VCC and metabolites can be analyzed and controlled automatically at-line with third-party analyzers integrated to the ambr systems.
Data such as monoclonal antibody (MAb) titers or cell counts can be collected automatically by ambr software and transferred to SIMCA multivariate data analysis (MVDA) software to build robust models for identifying critical process parameters (CPPs), optimizing bioprocessing conditions, and defining a robust design space. Combining multiparallel bioreactors with process optimization and integrated analytics makes them a logical choice as the minibioreactors for this study.
In this study, BIOSTAT STR bioreactors with single-use FlexSafe STR bags (Sartorius) were used for the pilot- and manufacturing-scale single-use bioreactors. They use proven and established design principles such as a top-driven, midpositioned stirrer and harmonized impeller and consistent sparger design across scales, with two impellers and a unique combination of ring spargers and microspargers. The technology allows for consistent mixing and gassing throughout bioreactor scales from 50 L to 2,000 L (10). As with ambr bioreactors, monitoring and analysis are achieved using process analytical technology (PAT) sensors with equivalent designs to those used in ambr 250 bioreactors. Data also can be transferred automatically to SIMCA MVDA software, ensuring equivalence of data generation and analysis across scales.
To overcome physical scale-up challenges such as changes in probe size relative to vessel size (which can interfere with fluid patterns) — and to move away from using scale-dependent parameters for scaling — we used a process-engineering approach. That helped to develop a scale-conversion tool based on algorithms using kLa, Newton-number, and specific power input (for example) to allow for scaling multiple parameters in a bioreactor independent of size.
In this study, we successfully used the scale-conversion tool for scaling parameters independent of bioreactor size to determine optimal agitation and gassing parameters for the selected single-use bioreactors. We implemented this in a proof-of-concept study to establish the possibility of scaling up a well-characterized commercial MAb production process from miniature single-use bioreactors (15 mL and 250 mL) and transferring that process from pilot-scale to 2,000‑L single-use bioreactors — all with a minimal number of transfer steps but without adversely affecting KPIs and CPPs.
Materials and Methods
Cell Line: Cellca CHO DG44 (Sartorius) cells expressing a human IgG1 MAb (used as a commercial biologic globally) were chosen to determine the effect on KPIs and CQAs while scaling a process from 15 mL to 2,000 L. We selected this cell line, with its associated media and cell-culture process, for a proof-of-concept study across all scales because the process and the biologic it produces are proven industrially and have been well-characterized at Sartorius laboratories in Germany, India, the United Kingdom, and the United States.
Optimizing Agitation: Before running scale-up studies in the single-use bioreactors, we used a scale-conversion tool created at Sartorius to determine the range of specific power inputs (P/V), impeller-tip speeds, and Reynolds Numbers (Re) required with respect to agitation rates. The scale-conversion tool is based on a set of algorithms developed using a full characterization of Sartorius single-use bioreactor systems and previous knowledge about critical environmental influences on the Chinese hamster ovary (CHO) cell line being studied. This tool was applied to a range of single-use bioreactors (15 mL to 2,000 L) to determine the most beneficial parameter set for optimal agitation to ensure homogenous mixing, cell suspension, and minimal shear-force damage to cells across scales.
Scalable Gassing Strategy: The kLa value for a scalable gassing strategy for efficient oxygen transfer has been determined previously for ambr, UniVessel, and BIOSTAT STR bioreactors (10) using the gassing-out method according to a DECHEMA guideline (11). Based on the agitation parameters determined by the scale-conversion tool, we can demonstrate scalable kLa values of 8 1/h by adjusting the air- and gas-flow rates used around a specific DO setpoint. For inoculation, nitrogen gas was sparged to adjust the DO to 60%. Then initial gassing rates were adjusted using nitrogen and air to maintain a kLa of 8 1/h. That allows for a comparable cell count across scales when oxygen addition begins.
Small-Scale Bioreactors: Two small-scale bioreactors were selected: the ambr 15 bioreactor (15-mL working volume) designed for clone screening, clone selection, and early process development; and the ambr 250 bioreactor (250-mL working volume) for process optimization and scale-up of cell culture. The ambr 15 bioreactor is suitable for early process-development studies and can mimic performance of benchtop bioreactors at 2-L to 7-L scale (12–14).
The ambr 250 vessel is particularly suited for developing a process for scale-up transfer because it has two pitched-blade impellers and an open pipe sparger. It offers geometrical similarities to the BIOSTAT STR pilot- and commercial-scale bioreactors with respect to H/D ratio and impeller dimensions (15). It has been shown in scale-up studies to generate comparable cell growth and protein titer profiles to those generated in 1,000-L bioreactors (16).
Benchtop Bioreactor: A UniVessel (5-L) glass benchtop bioreactor (Sartorius) also was used in this study because it was the reference vessel in which process development runs were performed previously with this cell line and process.
Commercial-Scale Bioreactors: Three scales of BIOSTAT STR single-use bioreactors were selected for this study: those with 50-L, 200-L, and 2,000-L maximum working volumes. Two other scales of single-use bioreactors (500 L and 1,000 L) are available and have been used in runs with the same CHO cell culture, but the data from those runs are not discussed here. All BIOSTAT STR single-use vessels have similar geometries across scales, maintaining an H/D ratio of 2:1 and an impeller-to-bag diameter ratio of 0.38 (17). The three scales shown represent the fewest transfer steps from process development through pilot to production scales. All vessels used FlexSafe STR bags with two three-blade segment impellers and a combisparger. Bags were quality tested for leaks at the point of use with a Sartocheck 4 Plus bag tester (Sartorius) according to an established published protocol (18).
Proof-of-Concept Process-Transfer Study: Cell culture scalability studies were performed using ambr 15, ambr 250, and BIOSTAT STR 50-L, 200-L, and 2,000-L bioreactors at Sartorius in Germany. Historical data from Sartorius UK, India, and the United States were used to compare process performance, KPIs, MAb titers, and CQAs across scales.
Before inoculation, conditions such as temperature equilibration of media and feed durations were chosen so that they were equivalent in all bioreactors. The production-stage BIOSTAT STR bioreactors were filled with production medium (PM) and equilibrated for a day before inoculation to ensure comparison between scales. A four-hour heat-up profile from ambient to process temperature of 36.8 °C protected the medium from overheating effects. The temperature set-point remained unchanged throughout the entire process.
Also before inoculation, the capacitance measurement in the BIOSTAT STR bioreactors (which use BioPAT ViaMass sensors) was set to zero picofarad (pF)/cm to ensure the same starting conditions at all bioreactor scales.
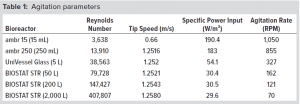 Because of different agitation and gassing principles of the ambr, UniVessel, and BIOSTAT STR vessels, process conditions were specified to meet a kLa value of 8 1/h. Critical process parameters were controlled according to the following setpoints:
Because of different agitation and gassing principles of the ambr, UniVessel, and BIOSTAT STR vessels, process conditions were specified to meet a kLa value of 8 1/h. Critical process parameters were controlled according to the following setpoints:
Temperature was 36.8 °C, pH 7.1, and DO 60%.
Bioreactor agitation rates were set according to the scale-conversion tool to correlate with Re (>3,000), tip speed (0.6–1.25 m/s), and a specific power input of 30–200 W/m³ across scales (Table 1).
The tip speed and Re in the ambr bioreactors are lower than the values obtained by the BIOSTAT STR reactor because those bioreactors have only one impeller, and the BIOSTAT STR reactors have two impellers. We applied a scalable gassing strategy as described above.
CHO DG44 cells expressing a human IgG1 MAb were cultured in all bioreactor types in a fed-batch process using stock culture medium (Sartorius) for the seed train and production medium (Sartorius) as the basal medium for fed-batch culture. The target cell concentration after inoculation was 0.2 × 106 cells/mL in the seed train and 0.3 × 106 cells/mL at the production stage. The individual seed-culture steps were three- to four-day cultures, whereas the first and last seed-culture step was a three-day seed culture with a target VCC of 2.5 × 106 cells/mL after three days. The initial volume after inoculation was maintained at 66% of the maximum working volume in all bioreactors.
After a three-day batch phase, a nine-day fed-batch regime was implemented using three different feeds (feed medium A, feed medium B, and a concentrated glucose solution). The automated discontinuous bolus feed of feed media A and B was supplemented by an on-demand glucose feed solution (400 g/L) to maintain glucose concentration above 3 g/L. For the 5-L bioreactors and BIOSTAT STR bioreactor, automated feed and glucose additions were made through a peristaltic pump; for the ambr 250 bioreactors, those additions were made through syringe pumps built into the ambr workstation; and for the ambr 15, they were made through the workstation’s automated liquid-handling system.
Sampling and Analysis: Daily samples were taken automatically for 12 days. DO and pH were measured by means of integrated optical single-use sensors in the ambr 15 bioreactors and FlexSafe STR bags; by using an integrated optical single-use DO sensor and electrochemical pH probe in the ambr 250 vessel; and by BIOSTAT A electrochemical sensors in the UniVessel bioreactor.
Metabolites such as glucose and lactate were analyzed by BioProfile FLEX2 automated cell culture analyzer system (Nova Biomedical) in ambr vessels and by the Radiometer ABL800 basic system (Radiometer, Germany) in the BIOSTAT STR and UniVessel reactors. VCC and cell viability were determined by BioPAT ViaMass in the BIOSTAT STR bioreactors or by a Cedex HiRes analyzer (Roche Diagnostics, Germany).
Osmolality was measured using the Osmomat 030 osmometer (Gonotec) or Cedex HiRes analyzer in the ambr 15 vessel and BioProfile FLEX2 automated cell culture analyzer system in the ambr 250 vessel. When capacitance measurements were conducted with the BioPAT ViaMass sensor, those data were acquired automatically for analysis.
Cell-free supernatant was sampled daily for analysis of expressed antibody titer and N-glycan profiling throughout all scales except for the ambr 15 scale, and the MAb titer was measured only on day 12 of culture. Profiling of titer and N-glycan was carried out by a commercial analysis service at Sartorius in Scotland using samples from the UniVessel bioreactor and BIOSTAT STR vessels. A LabChip GXII Touch protein characterization system (Perkin Elmer, Seer Green, UK) was used for the complementary analysis of the ambr bioreactor samples in Göttingen, Germany.
Reference Standard: A historical data set of golden-batch results was analyzed. Those data were gathered from cells cultured in 30 different ambr 250, UniVessel, and BIOSTAT STR bioreactor runs during which CHO cells expressing commercial IgG achieved peak VCCs >20 × 106 cells/mL and 80% cell viability at the day of harvest. From the resulting data, we used a mean of their KPIs and metabolite profiles to construct a reference standard.

Figure 1: Scaling-tool data showing the optimum Re, tip speed, and PPV based on agitation rate
Results and Discussion
Scale-Conversion Tool: Using a scale-conversion tool developed by Sartorius, a single strategy can be applied to the complete range of Sartorius single-use vessels from the ambr 15 minibioreactor all the way up to 2,000-L BIOSTAT STR bioreactors. Figure 1 shows how three different parameters — Re, tip speed, and P/V — changed according to agitation rate, with functions developed with a score from 0 to 1 over the range of the parameters, with 1 representing the optimum for each parameter.
With the scale-conversion tool, in this study a Reynolds number for turbulent flow of the liquid for homogeneous mixing (>3,000), tip speed of 0.6–1.25 m/s, and a specific power input of 30–200 W/m³ were set to generate the optimum cell culture conditions across all the single-use bioreactors.
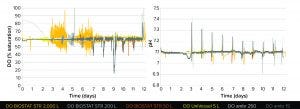
Figure 2 DO profile (left) and pH (right) of a Chinese hamster ovary (CHO) cell fed-batch culture over 12 days around a 60% DO
set point
Process Transfer Performance: As shown in Figure 2 (left), the DO profile across bioreactor scales is comparable, with all bioreactors showing DO oscillation of ≤30% around the DO set-point up to day 6 while CHO cells are in the exponential growth phase and before feed and glucose addition. After day 6, when cells are in the stationary phase and receiving feed and glucose additions, the DO is well controlled around a 60% setpoint with 5% changes in DO across all bioreactor scales except for the ambr 15 minibioreactors. Those show temporary decreases of 20–40% in the DO set-point. We think that the dips are caused by opening up the headspace for sampling, which produces a headspace exchange that is larger for the ambr 15 vessel than for the bigger ambr 250 and BIOSTAT STR bioreactors. Those vessels are not opened, so the headspace exchange does not change. For future studies, the DO profile might be improved with the BIOSTAT STR bioreactor by using newly available and precise mass-flow controllers (MFCs). Initial studies using the 2,000‑L BIOSTAT STR bioreactors with MFCs demonstrate that changing from solenoid valves to the new controller type improved DO control significantly (data not shown).
All the scales also show a similar pH profile (Figure 2, right), with changes of between 0.1 and 0.3 seen daily around the pH set-point of 7.1. Increases in pH are due to the addition of Feed B, which has a highly basic nutrient composition. Therefore the pH change is an expected and required event associated with feeding and is seen across all scales.
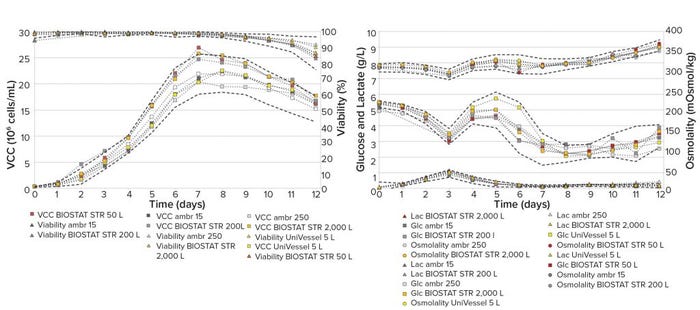
Figure 3: VCC and viability (left) and glucose, lactate, and osmolality profile (right) of CHO cells cultured over 12 days in bioreactor
scales from 15 mL to 2,000 L; dashed black lines represent historical data for each parameter measured, with +2 and –2 standard
deviations (SD).
Bioprocess Performance at Different Scales: The CHO cell culture showed comparable performance in cell growth, metabolic profiles, and product quantity/quality at all scales. Figure 3 (left) shows that the VCCs and cell viabilities have similar growth curves in all bioreactor scales, with peak VCCs on days seven and eight of 20–26 × 106 cells/mL and 95% viabilities and with VCCs of 15–18 × 106 cells/mL and 80–90% viability at the end of the process run. Figure 3 (right) shows that glucose, lactate, and osmolality profiles are similar across scales, with lactate levels peaking at 1 g/L on day 3 before feeds and glucose additions start. Lactate is consumed, and after day 3 it becomes depleted. Glucose levels are 3–5 g/L up to day 7 during the growth phase and 3–6 g/L from days 7 to 12. Osmolality increases steadily after day 7 and peaks at 350–375 mOsmo/kg, suggesting that viable cells in the culture are increasing in diameter after day 7. These results indicate that glucose, lactate, and osmolality profiles are transferable across different bioreactor scales and could be used as indicators for scale-consistent consumption of metabolites. The KPI results also compared well with the historic golden-batch reference data set.
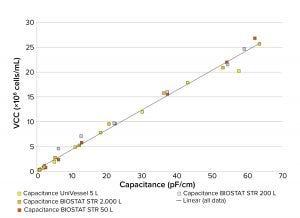
Figure 4: Linear regression model of capacitance and VCC of CHO cells cultured over 12
days in bioreactor scales from 5 L to 2,000 L
Scalability of On-Line Analytics: The linear regression capacitance model constructed using on-line BioPAT ViaMass data (Figure 4) and measured at a single frequency indicates transferability between scales during the exponential growth phase with an R2 value of 0.99, This finding is aligned with other published data (19). When the cells reach a stationary phase above 20 × 106, correlation is less well defined because of changes in cell size. Other studies using the BioPAT ViaMass system have suggested that CHO cells be analyzed by measuring capacitance across a range of frequencies (frequency scanning) to cope with changing cell diameter during this growth phase (20).
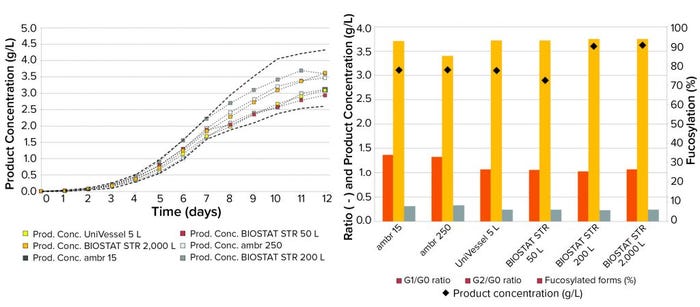
Figure 5: Daily product concentration (left) and cumulative product concentration, glycosylation, and fucosylation profiles (right) of a
MAb expressed by CHO cells cultured over 12 days in 15-mL to 2,000-L bioreactors; dashed black lines in Figure 5a represent historical
data +2 and –2 standard deviations.
MAb Titer and CQAs: Both daily (Figure 5, left) and total product concentrations (Figure 5, right) showed comparable trends across all scales with harvest concentrations of 2.5–3.5 g/L achieved on days 11 and 12. No CQAs of the MAb were affected during process transfer (Figure 5, right). Trends in glycosylation patterns of G2/G0 and G1/G0 ratios were similar, and binding potencies were comparable, with 85–90% fucosylated forms produced from cells cultured from the complete bioreactor range.
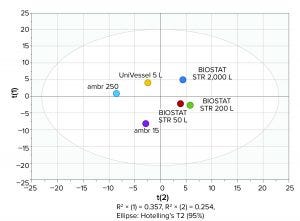
Figure 6: Batch-level model generated on the basis of a principal component analysis
(PCA) of bioprocess data from 15-mL to 2,000-L scale bioreactors
Principal Component Analysis: A principal component analysis (PCA) was run using SIMCA MVDA software with VCC, viability, glucose, lactate, osmolality, cell diameter, product quality, and final titer data from the ambr and BIOSTAT STR bioreactor runs. As Figure 6 shows, that analysis demonstrated that small- and large-scale batch data cluster closely together near the center of the plot, with data from the larger bioreactors clustering together on one side of the plot because of their generally higher VCCs and titers. All batch data fall within the 95% confidence region, indicating scalability of the process from 15 mL to 2,000 L.
Successful Scaling
The scale-conversion tool described above is a novel multiple-parameter scale-up approach to optimizing the agitation and gassing strategy for five different-sized single-use bioreactors. Bioreactors ranged from those for cell culture process development (ambr bioreactors), through pilot to manufacturing scale (BIOSTAT STR bioreactors) for volumes from 15 mL to 2,000 L. The success of this approach is based on a scientifically proven and established design adapted from traditional stainless-steel bioreactors, rolled out to the entire range of single-use bioreactors, and using very similar vessel geometry. Based on process optimization data and information from the scale-conversion tool, an industrially proven CHO process was performed using single-use bioreactors from 15 mL to 2,000 L to determine whether the process was scalable and could be transferred from pilot to manufacturing bioreactors in only a few steps. Data from the UniVessel bioreactor and historical golden-batch runs were used as a reference.
Our proof-of-concept study showed that DO and pH profiles in the CHO cell-culture process are comparable across scales from miniature to production bioreactors. Transfer of process parameters was achieved in less time through use of multiparallel, small-scale experiments. KPIs also demonstrated comparable performance across scales and were similar to reference data regarding cell growth, metabolic profiles, and cell diameter. Peak VCC in all bioreactor scales was consistently 20–26 × 106 cells/mL with 80–90% cell viability at harvest. Also, analysis of glycosylation patterns of the MAb produced in this proof-of-concept cell-culture process and PCA analysis showed comparable results throughout the complete bioreactor range, indicating that this process transfer approach could support reliable and robust MAb production at all scales.
On-line analytics also demonstrated good equivalence of the BioPAT ViaMass sensors when running single frequency measurements at the cells’ exponential stage. That indicates the possibility of using on-line VCC data for activities such as triggering inoculation or precise administering of feed to cells in an exponential growth phase. Using this sensor’s frequency-scanning function, online viable cell density analysis also could be extended into the stationary phase in pilot- and manufacturing-scale BIOSTAT STR bioreactors (20).
Using the new scale-conversion tool, miniature and multiparallel bioreactors for process development, and a three-step pilot-to-manufacturing scale-up approach potentially enables faster and more cost-effective manufacturing of biologics in fed-batch cell cultures. With our company’s new range of single-use perfusion bioreactors (scaled from 250 mL to 2,000 L) and optimized sparger concepts that prevent extensive foam generation, along with scalable PAT tools such as the BioPAT Spectro Raman spectroscopy tool, this approach also could be investigated to optimize and improve perfusion cell-culture performance.
Acknowledgments
We thank the global Sartorius R&D teams for generating the bioprocess data for this project. We thank Adrian Stacey at Sartorius Royston for providing the prototype of the scale-conversion tool and for his advice on its application, and Marek Hoehse at Sartorius Göttingen for generating PCA analysis plots.
References
1 Pohlscheidt M, et al. Avoiding Pitfalls During Technology Transfer of Cell Culture Manufacturing Processes in the Pharmaceutical Industry — Mitigating Risk and Optimizing Performance. Pharm. Outsourcing 14(2) 2013: 34–48; https://www.pharmoutsourcing.com/Featured-Articles/133770-Avoiding-Pitfalls-during-Technology-Transfer-of-Cell-Culture-Manufacturing-Processes-in-the-Pharmaceutical-Industry-Mitigating-Risk-and-Optimizing-Performance.
2 Xing Z, et al. Scale-Up Analysis for a CHO Cell Culture Process in Large-Scale Bioreactors. Biotechnol. Bioeng. 103(4) 2009: 733–746; https://www.ncbi.nlm.nih.gov/pubmed/19280669.
3 Löffelholz C, et al. Bioengineering Parameters for Single-Use Bioreactors: Overview and Evaluation of Suitable Methods. Chem. Ing. Tech. 85(1–2) 2013: 40–56; https://doi.org/10.1002/cite.201200125.
4 Xu S, et al. A Practical Approach in Bioreactor Scale-Up and Process Transfer Using a Combination of Constant P/V and vvm as the Criterion. Biotechnol Prog. 33(4) 2017: 1146–1159; https://doi.org/10.1002/btpr.2489.
5 Khattak S, Pferdeort V. Development and Qualification of a Cell Culture Scale-Down Model. Cell Culture Engineering: Recombinant Protein Production, Gyun ML, Kildegaard HF (Eds). Hoboken, NJ: Wiley (ebook), 2019: 391–405; https://www.wiley.com/en-us/g%3A+nt+Protein+Production-p- 9783527343348.
6 Manahan M, et al. Scale-Down Model Qualification of ambr® 250 High-Throughput Mini Bioreactor System for Two Commercial-Scale MAb Processes. Biotechnol. Prog. 17(6) 2019: e2870; https://doi.org/10.1002/btpr.2870.
7 Brunner M, et al. Investigation of the Interactions of Critical Scale-Up Parameters (pH, pO2 and pCO2) on CHO Batch Performance and Critical Quality Attributes. Bioprocess Biosyst. Eng. 40(2) 2017: 251–263; https://doi.org/10.1007/s00449-016-1693-7.
8 De Wilde D, et al. Superior Scalability of Single-Use Bioreactors. BioProcess Int. 12(8s) 2014: 14–19; https://bioprocessintl.com/upstream-processing/upstream-single-use-technologies/superior-scalability-single-use-bioreactors.
9 Market Alert Survey on Mini Bioreactors. Aspen Alert 7 August 2019; https://aspenxchange.com/2019/08/07/26305.
10 Dreher T, et al. Design Space Definition for a Stirred Single-Use Bioreactor Family from 50 to 2,000 L Scale. Eng. in Life Sci. 14(3) 2014: 304–310; https://doi.org/10.1002/elsc.201300067.
11 Meusel W, et al. Recommendations for Process Engineering Characterisation of Single-Use Bioreactors and Mixing Systems by Using Experimental Methods. DECHEMA Biotechnologie: Frankfurt, Germany: 2016; https://dechema.de/dechema_media/Downloads/Positionspapiere/SingleUse_ringCaracterisation_2016-p-20001485.pdf.
12 Hsu WT, et al. Advanced Microscale Bioreactor System: A Representative Scale-Down Model for Bench-Top Bioreactors. Cytotechnol. 64 (6) 2012: 667–678; https://doi.org/10.1007%2Fs10616-012-9446-1.
13 Nienow AW, et al. The Physical Characterisation of a Microscale Parallel Bioreactor Platform with an Industrial CHO Cell Line Expressing an IgG4. Biochem. Eng. J. 76, 2013: 25–36; https://doi.org/10.1002/elsc.20130006710.1016/j.bej.2013.04.011.
14 Lewis G, et al. Novel Automated Micro-Scale Bioreactor Technology: A Qualitative and Quantitative Mimic for Early Process Development. BioProcess. J. 9(1) 2010: 22–25; https://doi.org/10.12665/J91.Wales.
15 Bareither R, et al. Automated Disposable Small Scale Reactor for High Throughput Bioprocess Development: A Proof of Concept Study. Biotech Bioeng. 110(12) 2013: 3126–3138; https://doi.org/10.1002/bit.24978.
16 Xu P. Characterization of TAP Ambr® 250 Disposable Bioreactors, As a Reliable Scale-Down Model for Biologics Process Development. Biotechnol. Prog. 33(2) 2017: 478–489; https://doi.org/10.1002/btpr.2417.
17 Noack U, et al. Single-Use Stirred Tank Reactor BIOSTAT CultiBag STR: Characterization and Applications. Single-Use Technology in Biopharmaceutical Manufacture, Eibl R, Eibl D (Eds). Hoboken, NJ: Wiley, 2010: 225–240; https://doi.org/0.1002/9781119477891.
18 Hogreve M, et al. Integrity Redefined Consistent Robustness and Integrity Testing Lead to Enhanced Process Integrity and Patient Safety. BioProcess Int. 17(5) 2019: 51–55; https://bioprocessintl.com/sponsored-content/integrity-redefined-consistent-robustness-and-integrity-testing-lead-to-enhanced-process-integrity-and-patient-safety.
19 Metze S, et al. Monitoring Online Biomass with a Capacitance Sensor During Scale-Up of Industrially Relevant CHO Cell Culture Fed-Batch Processes in Single-Use Bioreactors. Bioprocess and Biosyst. Eng. 43(2) 2020: 193–205; https://doi.org/10.1002/978111947789110.1007/s00449-019-02216-4.
20 Metze S, et al. Multivariate Data Analysis of Capacitance Frequency Scanning for Online Monitoring of Viable Cell Concentrations in Small-Scale Bioreactors. Anal. Bioanal. Chem. 412(9): 2089–2102; https://doi.org/10.1007/s00216-019-02096-3.
Corresponding author Jens-Christoph Matuszczyk is the bioprocessing upstream manager at Sartorius, Göttingen, Germany, 49-551-308.4245, [email protected].
You May Also Like






