- Sponsored Content
- Cell Line Development
- Expression Platforms
Building the Next Generation of Cell-Line–Development PlatformsBuilding the Next Generation of Cell-Line–Development Platforms
Sponsored by Sartorius
Speed to market is a critical business driver in the biopharmaceutical industry. However, drug development success also requires building a robust process that maximizes efficiency while limiting the cost of goods. Achieving time and cost savings without compromising product quality is critical. Development of a productive and stable cell line is the foundation of an efficient and high-performing bioprocess.
Cell-line development (CLD) represents some of the most resource-intensive steps within a process development pipeline. Bioprocess scientists must find a balance between reducing timelines and costs while still carrying out comprehensive CLD activities to ensure the desired level of performance. Early investment in technologies and services to accelerate the search for a high-performing cell line can pay dividends later (1).
Novel automated and high-throughput single-cell cloning technologies are being introduced into CLD processes to meet those business needs (2). For example, accelerated single-cell cloning solutions and early prediction of high-producing clones help shorten timelines and increase productivity. Outsourcing CLD activities is one way to take advantage of such advances without significant investment in equipment and expertise.
As part of our continuous improvement, Sartorius scientists have upgraded our 4Cell CHO CLD platform to help solve our partners’ current and future challenges. Herein, we share how implementing pioneering selection technologies coupled with high-performing media allowed us to achieve optimal cell outgrowth, improve early recognition of high-producer clones, and reach expression titers of ~10 g/L. Perhaps most important, those innovations also allowed us to reduce the CLD timeline significantly, driving projects from DNA to research cell bank (RCB) to as little as nine weeks.
Next-Generation CLD
Figure 1 summarizes our 4Cell CLD workflow. The CHO DG44 host cell line is transfected stably with Sartorius’s proprietary expression vector carrying the gene of interest for a desired product. Single clones from the bulk pool are laid out in 24-nanowell plates and analyzed visually with the CellCelector system (Automated Lab Solutions, now part of Sartorius). The included software automatically collects data on cell characteristics and captures images demonstrating monoclonality. That information is evaluated alongside fluorescence parameters from a protein A bead productivity assay to identify high-producing clones. The device selects clones, transfers them to 384-well plates, and then expands them to 12-well plates.
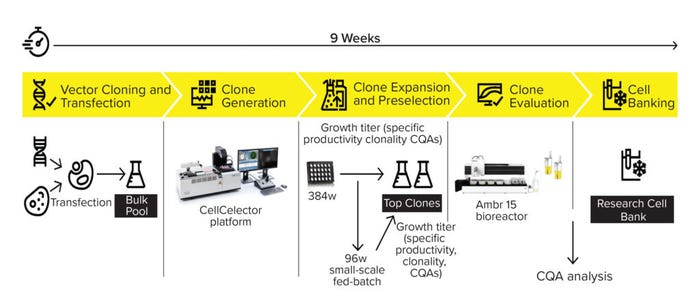
Figure 1: Overview of the new 4Cell CHO CLD process including the CellCelector system and the 96-well small-scale fed-batch process.
In parallel with the further expansion process (six-well plates, shake flasks), cells are inoculated in 96 wells of a deep-well plate to run a fed-batch culture. The 96-well fed batches serve as a scale-down model for an Ambr 15 bioreactor process and fine-tune the preselection of clones derived from the CellCelector system. Growth and productivity data from the small-scale fed batches are used to select and evaluate the top clones using an Ambr 15 device. The resulting data set, together with predefined critical quality attributes (CQAs), is used to define the top four clones for preparation of the RCB.
Key Features of an Optimized CLD Strategy
Below, we summarize the key elements of our optimized CLD platform that allow us to drive CLD projects from DNA to RCB in nine weeks and achieve titers of up to 10 g/L.
Reliable Single-Cell Cloning (SCC) and High-Producer Preselection: Achieving and demonstrating monoclonality are critical for meeting regulatory requirements. To meet those needs in our CLD workflow quickly and reliably, we used the CellCelector system as a highly automated solution for single-cell cloning. It has a robotic arm, a fluorescent microscope for cell isolation and visualization, and integrated software for automated analysis and early prediction of high producers. Extensive development and validation studies show that the device delivers clonality assurance with high reliability (99.7% probability of monoclonality).
The high-throughput capabilities of the CellCelector system allow us to use a bulk pool approach (which takes 10 days) instead of the previously used expansion and preselection of minipools (which takes 35 days), representing a significant timeline reduction. The high level of automation minimizes human error and enables the simultaneous screening of 10,000s of clones per run (Figure 2).
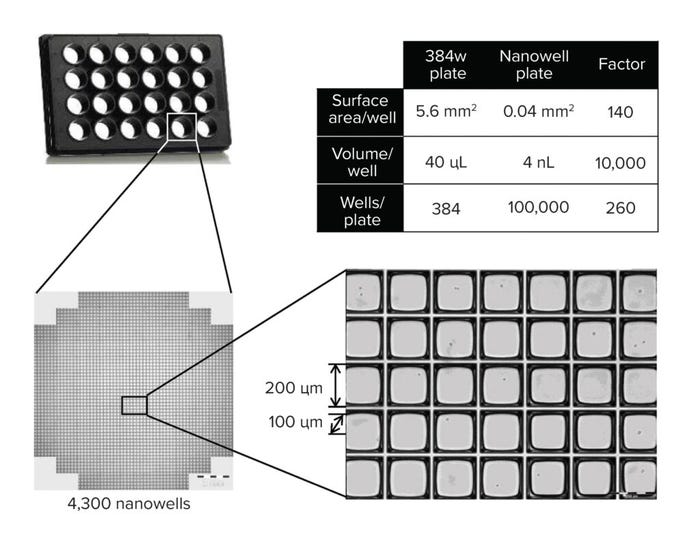
Figure 2: High-throughput screening in nanowell plates.
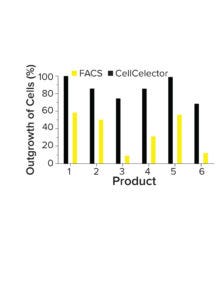
Figure 3: Comparing cone outgrowth in fluorescence-activated cell sorting (FACS) and a CellCelector-based selection.
Improved Clone Outgrowth: The precise and gentle isolation of cells by a CellCelector device improves the robustness of single-cell cloning. The device uses nanowell plates to facilitate crosstalk between cells and improve outgrowth (40–50% higher than FACS-driven cell selection) (Figure 3). Those features improve the reliability and efficiency of clone generation, even for complex products, minimizing the potential loss of high-producing clones because of poor outgrowth after single-cell cloning.
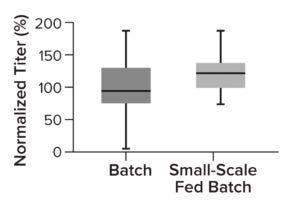
Figure 4: Comparing normalized titer of clones cultured in an Ambr 15 bioreactor when they are selected in batch cultures (shake flasks) with those of small-scale fed-batch cultures.
Two-Stage Clone Selection Strategy: Assessing clone performance in small-scale bioreactors ensures that results translate across scales. To fine-tune our clone-evaluation processes, we implemented an earlier selection step: small-scale (96-well plate) fed-batch cultures. They reflect later Ambr 15 bioreactor conditions, enabling quick identification and exclusion of low producers based on real-life process conditions (fed-batch culture). As a result, only the highest performing 24 clones are cultured further in an Ambr 15 bioreactor (Figure 4). That additional step increases the chance of finding high-producing clones early in a CLD process.
Optimized Cell Culture Media To Improve Performance: A high-performing medium is critical to supporting the growth and productivity of a cell line (3). We recently launched the 4Cell SmartCHO media system (an advanced formulation), and an upgraded version of the 4Cell XtraCHO system. Upgrades include improved media filterability, harvestability, and stability. The resulting cultures have higher cell-specific productivity, increased titers, and CQAs comparable to cells cultured in 4Cell XtraCHO media.
Genetic Engineering To Improve Product Quality and Efficacy: A common challenge with monoclonal antibodies (MAbs) is poor efficacy. Antibody engineering strategies are being used to modify CQAs that affect binding efficiency and potency and to improve clinical performance (4). One way to optimize those features is to manipulate the host cell metabolism genetically. For instance, MAb performance relies heavily on the ability to induce antibody-dependent cellular cytotoxicity (ADCC) and cause target cell death. However, MAbs typically are highly fucosylated, a posttranslational modification that limits their ability to stimulate ADCC (5). By knocking out the fut8 gene, we can create afucosylated MAbs with higher efficacy at lower doses (Figure 5). This service now has been added as a possible customization of our CLD workflow, helping our partners overcome product-quality challenges with minimal time lost. With such technologies and analytics in place, we also can address custom gene targets, facilitating custom optimizations.
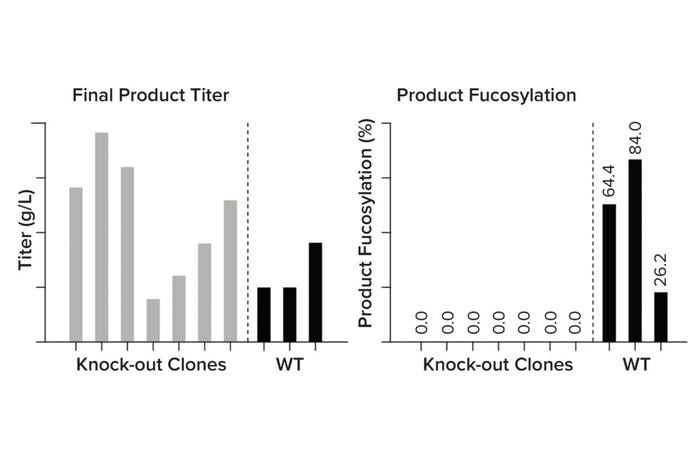
Figure 5: Comparing product titer and afucosylation in wild-type (WT) against fut8 knock-out clones.
Process Intensification By High- Inoculation Fed-Batch (HIFB): We use process-intensification strategies to increase the volumetric productivity of cell-culture processes. This approach allows us to achieve a higher expression titer (than standard fed-batch) in a comparable time or a comparable titer in a shorter time (Figure 6). N – 1 perfusion is used to grow cultures at high cell densities to inoculate a production bioreactor within six to eight days. This approach shifts biomass production from large-scale to a smaller prestage, enabling the most economical use of production facilities and saving costs. Our intensified processes are supported by 4Cell SmartCHOpe perfusion medium, a basal formula designed for intensified process formats.
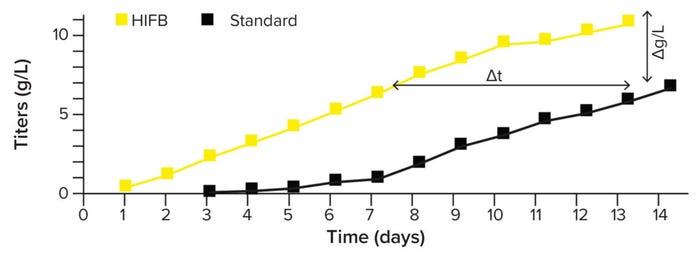
Figure 6: Product titer in standard and high-inoculation fed-batch (HIFB) cultures over 14 days.
Take-Home Message
Carefully designing a robust process that meets product-quality requirements and keeps timelines short are conflicting goals that place biopharmaceutical developers under significant pressure. Our next-generation CLD platform is the culmination of over 10 years of constant development and expertise following successful completion of >240 projects. By prioritizing speed, we reduced our project timelines and the transition from DNA to RCB to nine weeks. We do this without compromising performance: Our new technologies enable early prediction of clone performance to ensure that high producers are identified and expanded, achieving product titers of up to 10 g/L.
Our CLD platform also is versatile and suitable for a full range of modalities, including MAbs, bi- and multispecifics, Fc-fusion proteins, enzymes, hormones, and biosimilars. We are flexible to business needs, offering different tracks depending on budget and requirements. Because cells already are adapted to 4Cell SmartCHO media, no time-consuming media screening or optimization studies are required. Once a CLD process is complete, a cell line can be transferred to any contract manufacturing organization (CMO). The platform’s robustness allows cells to be scaled directly to larger volumes, eliminating time spent on scalability studies.
Get it right the first time: Explore Sartorius’s CLD services at https://www.sartorius.com/en/applications/biopharmaceutical-manufacturing/cell-line-development/cld-services.
References
1 Slingsby F, McLaughlin K. A Guide To Accelerating Cell Line Development for Commercial Production. Sartorius, 2021.
2 Tejwani V, et al. High-Throughput and Automation Advances for Accelerating Single-Cell Cloning, Monoclonality and Early Phase Clone Screening Steps in Mammalian Cell Line Development for Biologics Production. Biotechnol. Prog. 37(6) 2021: e3208; https://doi.org/10.1002/btpr.3208.
3 A Comprehensive Guide to Finding the Right Cell Culture Media for Your Bioprocesses. Sartorius, 2022; https://www.sartorius.com/en/ebook-cell-culture-media-comprehensive-guide-1273222.
4 Dai JM, et al. Modified Therapeutic Antibodies: Improving Efficacy. Engineering. 7(11) 2021: 1529–1540; https://doi.org/10.1016/j.eng.2020.06.030.
5 Yamane-Ohnuk N, Satoh M. Production of Therapeutic Antibodies with Controlled Fucosylation. mAbs 1(3) 2009: 230–236; https://doi.org/10.4161/mabs.1.3.8328.
Lisa Blaschke ([email protected]) is a product manager of cell line development services, Catherine Krikelis ([email protected]) is a product manager of cell culture media, and Joel Watkinson ([email protected]) is a product manager of cell banking and testing services at Sartorius. Katy McLaughlin ([email protected]) is a scientific content writer at Sartorius.
You May Also Like





