- Sponsored Content
- Filtration
Comparing Single-Use Multicycle Cake Filtration with Depth Filtration: Eliminating the Downstream BottleneckComparing Single-Use Multicycle Cake Filtration with Depth Filtration: Eliminating the Downstream Bottleneck
Sponsored by DrM
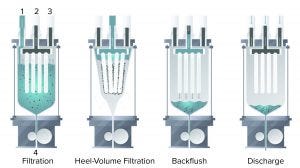
Figure 1: The four main steps of a Contibac SU filtration cycle (as defined in text, below)
Over the past few decades, single-use (SU) technology has increased bioproduction efficiency significantly, especially with the introduction of disposable bioreactors in upstream processing. To keep pace with major developments and increases in upstream capacity, downstream processes also must increase capacity and efficiency. However, cell harvesting and downstream processing continue to present bottlenecks in manufacturing (1).
Typical clarification processes are composed of primary and secondary clarification steps, such as centrifugation followed by depth filtration, respectively (1). Two sets of SU depth filters can be used for primary and secondary clarification, although they tend to foul quickly and must be replaced. Here we introduce the new Contibac SU filtration technology (Dr. Mueller AG, DrM) that combines primary and secondary steps in one unit operation. We compare that to an existing and well-known SU filtration technique — depth filtration — by evaluating flow rates, product yield, filtrate quality, laboratory footprint, and filtration area.
Filter Technology
Contibac SU technology is the only SU cyclical cake-filtration system available. Operating in cycles gives it backflush and filter-cloth regeneration capabilities, providing a novel way to prevent fouling — which is a common concern with other SU filtration options. When a SU filter clogs, it usually is considered to be spent and must be disposed of and replaced. The cyclical nature of the Contibac SU system provides users with the benefits of disposable technology while also prolonging the lifespan of one filter cloth for up to 200 cycles. Key to this cyclical filtration is the addition of diatomaceous earth, which combines with cells to form a porous cake. The cake actually performs the filtration while the filter itself chiefly acts as a support for it. Unlike in a depth filter, the filter medium thus can be coarse and thin so as to not trap debris over time.
Figure 1 shows the four main steps of a Contibac SU filtration cycle. Four filtration elements are each surrounded by a filter cloth. The combination is surrounded by a plastic film, creating a sterilizable 30-L bag. The bag is connected to corresponding inlets (1, 3), a filtrate outlet (2), and discharge hoses (4) and is sealed air-tight relative to the transparent vessel housing it.
In the first step, cell culture fluid is pumped into the bag and filtered, creating a porous cake of solids on the elements. Once filtration has ended — typically determined by a limiting factor of pressure or cake thickness — heel-volume filtration is executed by introducing pressurized gas to the area in between the housing vessel and the plastic bag. That squeezes the remaining liquid through the filter elements and out through the filtrate line. Cake washing is an optional step to ensure that product remaining in the cake can be recovered. At this point, the filter cloth is regenerated by pumping a sterile liquid such as wash buffer, water for injection (WFI), or filtrate back in the opposite direction through the filter elements to dislodge the cake and other fine particles that may have become embedded in it. Finally, the discharge valve opens so that remaining liquids and solids are discharged from the bag. This cycle can repeat 100–200 times to accommodate batch sizes of 2,000 L with one bag (2).
Experimental Methods
Results described below were obtained at laboratory scale using a 200-mL Nutsche filter operated at a constant pressure of 1.5 bar. Diatomaceous earth (Celpure C300 from Advanced Minerals Corporation) was used as filter aid in various ratios to wet cell weight (WCW). The operating principles of Nutsche and Contibac SU filters differ, but their flow rates are equivalent, which is why the results are labeled as Contibac SU throughout. The same pharmaceutical-grade filter cloth was used with both filters, and the backflush and filter-regeneration steps were simulated by turning a used filter cloth upside down and flushing it with water.
Samples measured for both the Contibac SU and depth-filtration data in Figure 2 came from Chinese hamster ovary (CHO) cell cultures that exhibited comparable total cell density and viability. Depth-filtration data came from existing literature (3, 4). To provide a realistic comparison with depth filters, the Contibac SU downtimes were estimated, yet reasonable.
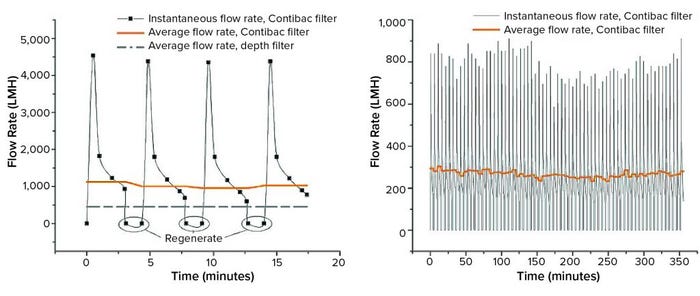
Figure 2: (left) Chinese hamster ovary (CHO) cell culture with a density of 23.5 million cells/mL and 87% viability was filtered through a Nutsche filter with 55% filter aid per wet cell weight (WCW). A realistic downtime of 75 seconds was estimated. The average flow rate is compared with data from depth filtration of CHO culture with a cell density of 24 million cells/mL at 65% viability using a MilliporeSigma Clarisolve filter (3). (right): 90 ExpiCHO cells at a cell density of 90 million cells/mL and 91% viability were filtered in a Nutsche filter using 40% filter aid per WCW; estimated downtime was again 75 seconds.
Results and Discussion
With cyclical function and cloth regeneration, a Contibac SU filter can maintain high flow rates, exemplified in Figure 2 (left). As indicated by the instantaneous flow rate, initially there is low resistance to the flow. As the cake builds over time, resistance grows, and the flow rate drops. At that point the cloth is regenerated, and the flow rate returns to a high peak value, showing low resistance. Cloth fouling over time is a serious concern for all SU filters, especially depth filters. Returning the flow rate to previous peak values following Contibac SU regeneration eliminates the concern of cloth fouling over time, demonstrating the power and effectiveness of this technology.
Integrating over the instantaneous flow curves and considering downtime, Figure 2 (left) shows that an average flow rate of 1,030 LMH was obtained for the Contibac SU at crude pH, which is over twice as fast as that for depth filters (measured at 450 LMH). That unequalled speed can be increased even further by reducing pH before filtration because the resulting aggregation of DNA, host-cell proteins (HCPs), and other impurities improves the filtration process (5). When culture pH at the inlet was reduced to 4.9 before Contibac SU filtration, the flow rate increased up to 100% depending on the percentage of filter aid used.
Furthermore, Contibac SU filters can handle much higher inlet turbidity than depth filters can. A CHO culture of 90 million cells/mL density with a WCW of 173.5 g/L was filtered at an average flow rate of 263 LMH. There is no comparison to the average flow rate achieved with depth filters because their fouling tendencies prevent them from offering a practical solution for processing suspensions at such high cell densities. By contrast, after 68 cycles of filtering an extremely concentrated culture, the Contibac SU filter exhibited no fouling. The cloth was regenerated to full capacity after each backflush step (Figure 2, right).
The Contibac SU filter’s significantly higher flux and capacity than those of depth filters could be attributed both to the regeneration step and to use of a proprietary filter cloth. Compared with depth-filter media, the Contibac SU filter cloth is much coarser and thinner, which helps keep flow rates high and product loss low. Whereas product can get stuck in thick and intricate 3D depth-filter media (6), Contibac SU product recovery is nearly 100% at crude pH. Meanwhile, the filter can perform excellent clarification with added filter aid.
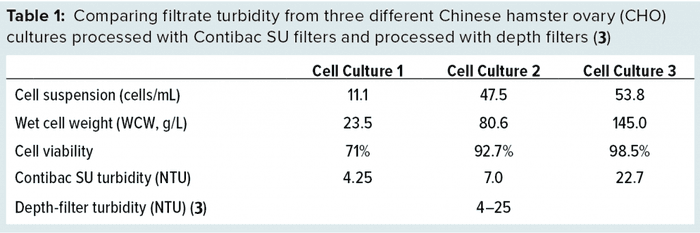 Although the relatively coarse and thin cloth of a Contibac SU filter allows for superior flow rates, it is important to note that filtrate quality is not compromised. The filter aid causes a porous cake to build up on the coarse cloth, aiding in clarification. As Table 1 shows, both Contibac SU and depth filters achieve filtrate turbidity values that are within the range of 4–25 NTU (3). Inlet suspensions of higher cell density lead to increased filtrate turbidity, as expected with a large number of impurities to filter out.
Although the relatively coarse and thin cloth of a Contibac SU filter allows for superior flow rates, it is important to note that filtrate quality is not compromised. The filter aid causes a porous cake to build up on the coarse cloth, aiding in clarification. As Table 1 shows, both Contibac SU and depth filters achieve filtrate turbidity values that are within the range of 4–25 NTU (3). Inlet suspensions of higher cell density lead to increased filtrate turbidity, as expected with a large number of impurities to filter out.
Space and size also are important aspects to consider when evaluating equipment. Scaling up a depth-filtration process requires elements to be stacked on top of each other to accommodate larger batches. Not only does that take up a larger laboratory footprint, but it also uneconomically generates a significant amount of waste because the filters must be disposed of once they have become fouled, consequently driving up operating costs. It can take 35 depth-filter capsules to process a 1,000-L batch of 25 million cells/mL (7), and even a batch at merely 14 million cells/mL requires a filter area of almost 5 m2 (4).
Alternatively, the Contibac SU technology accommodates batches from 200 L up to 2,000 L with the same bag (a filter area of 0.88 m2) and laboratory footprint — so the only parameter to adjust is the number of cycles that are executed. Because we regenerate the filter cloth after each cycle, the overall filtration area needed is much lower than that for depth filtration (4). Reducing the amount of filter material contacted by a fluid suspension lowers the potential for extractables and leachables to compromise a batch. In addition to the reduction of necessary filter material, the Contibac SU filter cloth is much thinner than depth-filter media, which also lessens product contact.
High Throughput and Quality
As bioprocessing gravitates toward SU technologies, and upstream efficiencies continue to improve, downstream developments must keep up to prevent the bottlenecks created by traditional filtration techniques. Although depth filters are used widely throughout the industry, they exhibit relatively low flow rates and cannot keep up with the demand for filtering highly concentrated cell solutions. Contibac SU multicycle cake-filtration technology diminishes downstream bottlenecks by processing high cell densities effectively with product yields close to 100%. In addition, a backflushing step regenerates the Contibac SU filter cloth, virtually eliminating fouling concerns. That reduces the necessary filter area, which in turn limits the potential for extractables and leachables. Furthermore, the equipment footprint does not change with scale-up.
Contibac SU technology can handle high cell concentrations without the risk of fouling and uses only one disposable bag per batch of up to 2,000 L. Quality and quantity are achieved simultaneously, with users finding exceptional filtrate turbidity at over twice the speed of depth filtration.
Acknowledgments
The authors thank Dieter Eibl and his team at Zurich University of Applied Sciences in Switzerland for providing the CHO cells used in the study reported herein.
References
1 Scott C. Downstream Disposables: The Latest Single-Use Solutions for Downstream Processing. BioProcess Int. 15(1)i 2017: 1–5; https://bioprocessintl.com/downstream-processing/downstream-single-use-technologies/downstream-processing-single-use-solutions.
2 Bucher T, et al. Cell Harvest with Contibac SU Filters. Dr. Mueller AG. May 2020: 1–8; https://drm-lifescience.com/cell-harvest-with-contibac-su-filters.
3 Sharma M, et al. Examining Single-Use Harvest Clarification Options: A Case Study Comparing Depth-Filter Turbidities and Recoveries. BioProcess Int. 15(2) 2017: 40–47; https://bioprocessintl.com/downstream-processing/filtration/case-study-comparing-depth-filter-turbidity-recoveries-examining-single-use-harvest-clarification.
4 Schmidt, Wieschalka S, and Wagner R. Single-Use Depth Filters: Application in Clarifying Industrial Cell Cultures. BioProcess Int. 14(1)i 2017: 6-11; https://bioprocessintl.com/downstream-processing/filtration/case-study-comparing-depth-filter-turbidity-recoveries-examining-single-use-harvest-clarification.
5 Minow B, et al. High–Cell-Density Clarification By Single-Use Diatomaceous Earth Filtration. BioProcess Int. 12(4) 2014: 36–46; https://bioprocessintl.com/2014/highcell-density-clarification-by-single-use-diatomaceous-earth-filtration-351074.
6 Challener C. Cell Harvesting Steps Separate the Good from the Bad. BioPharm Int. 33(10) 2020: 28–30; https://www.biopharminternational.com/view/cell-harvesting-steps-separate-the-good-from-the-bad.
7 Stax mAx Clarification Platform. Pall Biotech: Port Washington, NY, 2018; https://shop.pall.com/us/en/biotech/depth-filtration/stax-max-clarification-platform-zidht5ftm7f.
Corresponding author Chloe Jerrold‑Jones is a technical sales specialist for single-use technologies, and Tizian Bucher is an R&D engineer at DrM, Dr. Mueller AG, Alte Landstraße 415, 8708, Männedorf, Switzerland; [email protected]; 1-704-388-5409.
Contibac® is a registered trademark of DrM (Dr. Mueller AG).
You May Also Like





