- Sponsored Content
- Chromatography
- Separation/Purification
Development of a Universal Preparative Anion-Exchange Method to Purify OligonucleotidesDevelopment of a Universal Preparative Anion-Exchange Method to Purify Oligonucleotides
Sponsored by Tosoh Bioscience
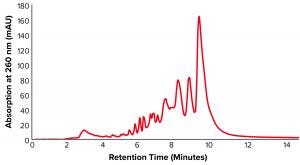
Figure 1: Anion-exchange high-performance liquid chromatography (AEX-HPLC) chromatogram of unpurified oligonucleotide sample with 42.17% purity
Oligonucleotide-based therapeutics have been studied over recent decades, and their promise as a new drug modality is now being realized. The growing interest in oligonucleotides is driven by their high potential for treating different medical conditions, the growing number of oligonucleotide drugs approved by the US Food and Drug Administration (FDA), an increased focus on personalized medicines, the development of therapies for rare diseases, and the wide adoption of nucleotide-based COVID-19 vaccines.
Oligonucleotides are short, linear sequences of DNA or RNA that generally are manufactured by chemical synthesis. Oligonucleotides are extremely susceptible to oxidation, enzymatic degradation, and clearance in vivo. Because of that, synthetic oligonucleotides often are chemically modified to improve their stability and make the molecules resistant to extracellular and intracellular nuclease degradation. One original and still the most widely used modification is the phosphorothioate modification of the oligonucleotide backbone.
Because of errors that may occur during oligonucleotide synthesis, nucleosides can be either missing (N – X) or attached in excess (N + X). The chirality of sulfur atoms in the backbone of oligonucleotides (caused by phosphorothioate modification) also leads to diastereomers. To remove those impurities, biopharmaceutical manufacturers rely on chromatography. Increasing demand for oligonucleotides necessitates a cost-effective and easy scale-up from research to commercial capacities.
We developed a universal method for purifying phosphorothioate oligonucleotides using TSKgel SuperQ-5PW (20), a resin designed specifically to provide high resolution and selectivity. This method can be a starting point in a laboratory to develop efficient, large-scale preparative processes. Before addressing purification, we developed an anion-exchange high-performance liquid chromatography (AEX-HPLC) analytical method using a TSKgel DNA-NPR column to assess purity and recovery.
UHPLC Analysis of Phosphorothioate Oligonucleotides
The crude oligonucleotides were analyzed before and after purification with analytical anion-exchange HPLC (AEX-HPLC) using a linear salt gradient over 20 minutes on a TSKgel DNA-NPR column (Figure 1). Other parameters were as follows:
TSKgel DNA-NPR (4.6-mm ID × 7.5-cm L) column
Thermo Fisher Dionex Ultimate 3000 ultrahigh-performance liquid chromatography (UHPLC) system
mobile phase A = 10 mM NaOH at pH 12; B = 2.0 M NaCl
flow rate = 0.5 mL/min
gradient = 0–100 %B in 20 minutes
detection = UV at 260nm
injection volume = 10 µm
sample = ssDNA full phosphorothioate (purity = 42.17%).
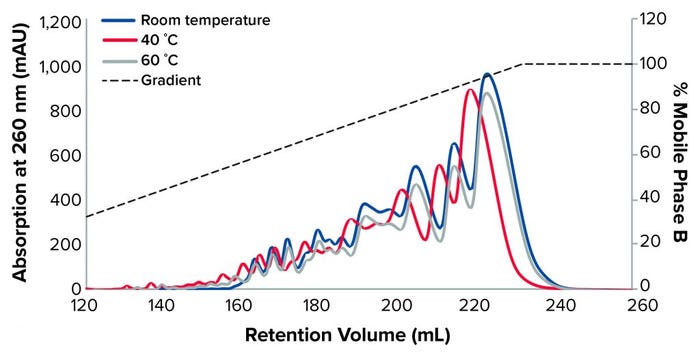
Figure 2: TSKgel SuperQ-5PW (20), 1-mg load at different temperatures
Purification of Oligonucleotides By AEX
We purified an ssDNA full phosphorothioate oligonucleotide with 42.17% purity after synthesis. To develop a robust preparative method for purifying oligonucleotides, we tested the performance of the TSKgel SuperQ-5PW resin at a 1-mg/mL loading at different temperatures and buffer compositions.
Material and Methods: The following parameters were applied:
column dimensions = 0.66-cm ID × 20-cm L
mobile phase A = 10 mM NaOH, pH 12 (and additives); mobile phase B = mobile phase A + 2 M NaCl
additives = 5% acetonitrile or 10 mM NaCl
flow rate = 1.2 mL/min
detection = UV at 260 nm
sample = 20-mer ssDNA full phosphorothioate (42.17% purity)
loading = 1 mg/mL resin.
Figure 2 shows the separation of the oligonucleotide at room temperature, 40 °C, and 60 °C with a NaOH buffer as the mobile phase and NaCl as the elution phase.
We observed a better resolution at 60 °C, as confirmed by AEX-HPLC (Table 1). Therefore, all subsequent experiments were performed at 60°C.
 Addition of chaotropic reagents or solvents reduces hydrophobic secondary interaction and can improve resolution in the purification of phosphorothioates, which are more hydrophobic than conventional phosphodiester oligonucleotides. The addition of low concentrations of salt to the binding buffer, reduces the ion exclusion effect. This leads to stronger binding of the sample and can potentially also increase resolution. To verify those assumptions, we evaluated the addition of 5% acetonitrile and 10 mM NaCl to the mobile phase.
Addition of chaotropic reagents or solvents reduces hydrophobic secondary interaction and can improve resolution in the purification of phosphorothioates, which are more hydrophobic than conventional phosphodiester oligonucleotides. The addition of low concentrations of salt to the binding buffer, reduces the ion exclusion effect. This leads to stronger binding of the sample and can potentially also increase resolution. To verify those assumptions, we evaluated the addition of 5% acetonitrile and 10 mM NaCl to the mobile phase.
Figure 3 shows the chromatograms on TSKgel SuperQ-5PW at a load of 1 mg/mL with the different buffer conditions at 60 °C. The addition of NaCl suppressed ion exclusion during binding, and the sample bound stronger to the resin, resulting in a later elution time. By contrast, adding acetonitrile decreased the retention time because it suppressed hydrophobic interactions with the stationary phase. For our tested sample, both additives lowered purity and recovery (Table 1).
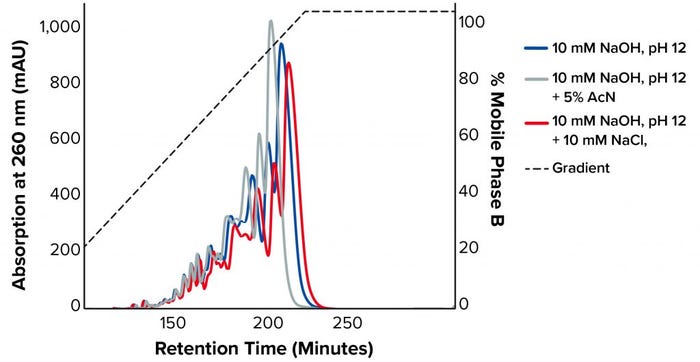
Figure 3: Chromatograms on TSKgel SuperQ-5PW at a load of 1 mg/mL with the different buffer conditions at 60 °C (AcN = acetonitrile)
An Improved Large-Scale Purification Method
We investigated the influence of temperature and buffer composition for the purification of oligonucleotides on the AEX resin TSKgel SuperQ-5PW (20). By increasing the temperature from room temperature to 60 °C, we could separate the impurities from the target oligonucleotide with higher resolution, recovery, and purity. Mobile-phase composition also has a significant influence on the purification of oligonucleotides. In our case, adding acetonitrile and NaCl to the equilibration buffer had a negative effect on recovery and purity.
Therefore, we recommend purifying oligonucleotides on TSKgel SuperQ-5PW (20) at elevated temperature, using a 10 mM NaOH buffer at pH 12 and NaCl as eluent. Several of our biopharmaceutical partners already have implemented similar methods for purifying oligonucleotides at relatively large scales, leading to improved process efficiency.
Romain Dabre ([email protected]) is senior product manager, and Patrick Endres ([email protected]) is junior manager of application development at Tosoh Bioscience. Hannah Bartonek is an intern at Tosoh and a graduate student in separations and purification sciences at Technische Universität Darmstadt in Germany.
You May Also Like





