- Sponsored Content
Efficient Removal of Endotoxins and Viruses By Anion-Exchange ChromatographyEfficient Removal of Endotoxins and Viruses By Anion-Exchange Chromatography
Sponsored by Tosoh Bioscience
 Various unit operations are needed in downstream processing (DSP) of biologics to remove process‑related impurities such as host‑cell proteins, nucleic acids, viruses, and endotoxins. This application note describes the potential of a salt‑tolerant anion‑exchange resin to achieve outstanding clearance of endotoxins and viruses.
Various unit operations are needed in downstream processing (DSP) of biologics to remove process‑related impurities such as host‑cell proteins, nucleic acids, viruses, and endotoxins. This application note describes the potential of a salt‑tolerant anion‑exchange resin to achieve outstanding clearance of endotoxins and viruses.
Introduction
Endotoxins are lipopolysaccharides (LPS) originating from cell membranes of Gram‑negative bacteria. They are frequent contaminants of recombinant proteins produced in microbial expression systems. On the other hand, expression of more complex proteins in eukaryotic systems carries an inherent risk of introducing adventitious viruses.
TOYOPEARL NH2‑750F, a salt‑tolerant anion‑exchange resin, is ideal for intermediate purification of monoclonal antibodies (mAbs) and other proteins. Aggregates and negatively charged impurities, such as DNA, viruses, and endotoxins can be removed from a target of interest.
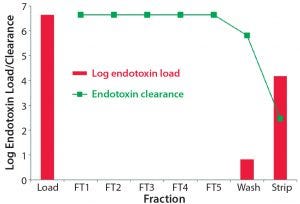
Figure 1: Log endotoxin clearance for each step and the corresponding fractions
Viral Clearance
In a virus validation study, four different viruses, namely murine leukemia virus (MuLV); Pseudorabies virus (PRV), reovirus type 3 (Reo), and minute virus of mice (MVM) were spiked in a post protein A sample of an antibody (765 mg mAb in 69.6 mL) and applied to a 6.6 × 150 mm (5.1 mL) TOYOPEARL NH2‑750F column (150 mg mAb/mL resin). Samples from the product pool were collected and analyzed using qPCR (infectious and noninfectious virus) and/or cell‑based infectivity assay (infectious virus). Log reduction values of TOYOPEARL NH2‑750F exceeded five for all of the four viruses (Figure 1).
Endotoxin Removal
To examine the endotoxin clearance potential of a flow through (FT) anion‑exchange step, a solution of Escherichia coli LPS was used to spike the loading buffer with an endotoxin content of about 100,000 EU/mL. A direct assay of this solution gave a starting concentration of 89,000 EU/mL, which was applied to a TOYOPEARL NH2‑750F MiniChrom column (8 × 100 mm, 5‑mL resin). Endotoxin clearance for a given fraction was determined by subtracting the log endotoxin content of the fraction from the log endotoxin content of the load.
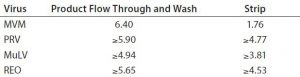
Table 1: Virus log reduction values for four viruses
There was some minor breakthrough of endotoxin during the wash phase, representing a breakthrough of <0.0002% of endotoxin from the original load material. The endotoxin concentration of the FT fractions that would contain the target molecule in a real purification scenario was less than the limit of detection for the endotoxin assay (0.1 EU/mL; Limulus amebocyte lysate (LAL) assay). The log reduction value for each FT fraction was >6.7 (Table 1).
Conclusions
The unique selectivity of TOYOPEARL NH2‑750F resin allows for its use in multipurpose chromatography steps. The anion‑exchange resin is very effective for removing endotoxins in FT chromatography mode. We observed excellent virus clearance capabilities. The resin also can be used to remove aggregates in the purification of therapeutic antibodies.
Regina Roemling is senior marketing manager, and Judith Vajda is a senior lab specialist at Tosoh Bioscience GmbH, Im Leuschnerpark 4, 64347 Griesheim, +496155 7043700, [email protected], www.tosohbioscience.de.
You May Also Like





