- Sponsored Content
- Facility Design/Engineering
- Contract Services
Facility Design: A Guide to Maximizing ValueFacility Design: A Guide to Maximizing Value
May 21, 2021
Sponsored by Sartorius
Rapid growth and changing market dynamics in biologics and vaccine sectors have prompted biopharmaceutical companies and contract development and manufacturing organizations (CDMOs) to build new manufacturing facilities at unprecedented speeds. This often means developing single-use (SU) or hybrid bioprocessing facilities with reusable legacy equipment for production of monoclonal antibodies (MAbs), antibody–drug conjugates (ADCs), and viral-based vectors.
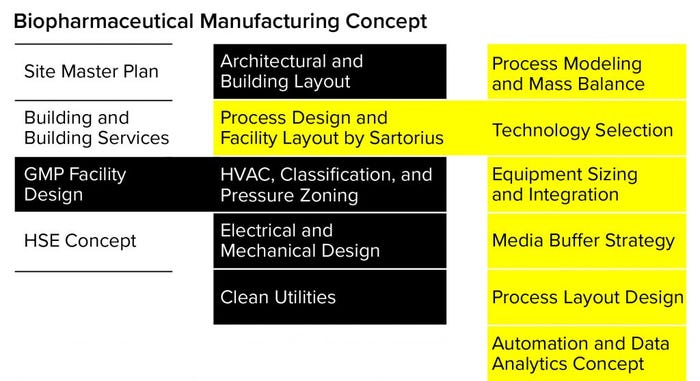
Figure 1: Work packages of a biopharmaceutical manufacturing concept highlighting process design and facility layout (HSE = health, safety, and environment)
For many companies, facility design begins by engaging an engineering consultancy to develop a layout that maximizes space use and minimizes equipment, fittings, and operating costs. SU technology suppliers are seldom consulted during the planning stage. Users assume that fitting SU equipment will not present a key facility design challenge, so they give more weight to other factors early on. However, involving SU suppliers at this stage helps companies resolve several issues more easily. For example, a supplier’s expertise in process design and SU technologies can improve accuracy about estimated levels of integration and space required by SU equipment. Tapping into SU suppliers’ extensive knowledge of their own technologies also can help with planning buffer and facility waste management and equipment qualification and validation. Such an approach helps ensure that a company will not face costly design and installation changes when SU equipment arrives on site.
SU technology suppliers that offer end-to-end upstream and downstream bioprocessing equipment and consumables have wide-ranging knowledge and understanding of their technologies in relation to facility design needs. They often can provide a design consultancy service with a process-centric approach to facility layout (1, 2). Leveraging a supplier’s SU expertise in a process and technology setup adds a risk- and science-based approach to design activities and can improve the accuracy of estimated operating costs and costs of goods (CoGs) for different modalities.
Sartorius is one of the few SU technology suppliers that offers facility design consulting within the range of its comprehensive services. The team of experts discuss both business and process needs with clients before suggesting facility layouts. In addition to factoring in throughput requirements, design suggestions incorporate facility type (greenfield or brownfield), whether it will be a single- or multi-product facility, and whether it will cater to a global or local biopharmaceutical market.
Good design consulting also considers the need to adhere to fixed processes so as to prevent regulatory refilings. This approach considers the type of products the facility is going to manufacture and whether the bioprocessing will include fed-batch, intensified, or continuous processing elements. During the layout planning, a good design also will incorporate room classification and segregation philosophies as well as the flow of personnel, materials, products, and waste throughout the facility (Figure 1).
Since 2016, Sartorius has offered a facility design service known as the Process4Success solution, which works collaboratively with customers and their engineering and construction partners to help deliver a process-centric facility layout (Figure 1). Using this strategy, Sartorius has helped more than 50 biopharmaceutical companies and CDMOs by providing or reviewing designs for new SU and hybrid good manufacturing practice (GMP) manufacturing facilities.
Defining Space and Cost
In 2019, biopharmaceutical company RemeGen, needed a new facility for GMP manufacturing of MAbs and ADCs to treat autoimmune diseases. As Michael Koch (head of sales for Applied Bioprocessing Integrated Solutions at Sartorius) explains, “For all biopharma companies, space is money. So all our customers want their manufacturing footprints to be as small as possible, but as big as necessary. RemeGen turned to us to provide a design concept for its new facility because we are a trusted partner that has worked with them for many years. Because we have a deep understanding of local National Medical Products Administration (NMPA) regulations and expectations from auditors, RemeGen knew that we would provide optimal design on layout.”
Charles Li (vice president of RemeGen and chief engineer of Rongchang Group) comments: “During the design process, Sartorius sent us the most advanced design concepts in the world, such as the widespread use of SU technology, intensified production technology, process analytical technology (PAT) data analysis, and other advanced technology applications. Sartorius also provided us with an excellent process layout, a design plan of the company, and constructive opinions about the levels of intelligent manufacturing, energy saving, and environmental protection. Through the combination of information and automation technology, the production process flow has been greatly optimized, and the production efficiency has been improved.”
By modeling multiple process scenarios, the Sartorius team delivered a design review in 14 weeks that showed RemeGen that its original 2,000-m2 plan was much too small for its process needs. The final design approved by RemeGen has an 8,000-m2 layout over two floors consisting of end-to-end upstream and downstream SU equipment. The facility will house 34 SU bioreactors with production capacity of up to 68,000 L and will be compliant with all regulations. Koch states, “If RemeGen had gone with its original plan of a 2,000-m2 facility, that could have caused some major issues with fitting out the plant, which down the line may have been difficult and costly to resolve.” In November 2020, RemeGen triggered a US$515 million initial public offering (IPO), one of the world’s largest IPOs for a biotechnology company, to help pay for the new facility (3), which is slated for completion in 2025.
As Li concludes, “We would like to thank Sartorius for introducing the world’s most advanced biopharmaceutical experience into the RemeGen industrialization project. This has laid a solid foundation for our later detailed design and construction of the project.”
Working in Partnership
The Swiss Biotech Center, a CDMO that supports start-ups with biopharmaceutical development and manufacturing plans, wanted to implement a SU pilot plant. The project was fraught with issues that needed an expert SU technology supplier capable of designing and installing SU equipment in an extremely limited classified environment under CGMP conditions. As Massimo Nobile (CEO of the Swiss Biotech Center) explains, “Our project was extremely ambitious, if not impossible according to some suppliers. From the start, Sartorius understood us and helped us in this process until we obtained authorization from our regulatory authorities to produce APIs in 2019. Their expert team has always sat on our side of the table, not taking a position as a supplier, but contributing as a partner. In our expansion plan, we see Sartorius as our key partner in changing the world of drug-substance production.”
More recently, CDMO Matica Biotechnology, Inc. wanted to build a viral-vector process development and clinical production facility in Texas, USA. Michael Koch, Sartorius, comments that “Matica Bio’s new biomanufacturing plant in Texas is designed to streamline scale-up and transfer from development to GMP manufacture. It is critical to align equipment platforms and processes with facility fit to mitigate technical risks during the production of complex advanced therapies. To achieve this, Sartorius worked together with the Matica Bio team and its engineering consultancy on review of its architectural plans.”
The Sartorius team modeled multiple process scenarios and suggested appropriate SU technology and an automation strategy for the existing layout. Koch notes that “in a review that took just two months, the team updated the facility layout to ideally accommodate the in-process and end-to-end upstream and downstream SU equipment.” Matica Bio’s new facility is now under construction using this new layout and is scheduled to open by the end of 2021 (4).
Michael L. Stewart (Matica Biotechnology’s chief technology officer) states that “working with Sartorius’s design team collectively, we settled on a reduction in equipment required and an increase in facility throughput. We are extremely proud of our partnership with Sartorius and will continue to work closely with them as we expand capabilities to bring cutting-edge, on-line, real-time technologies and add new modalities to our portfolio of services.”
Consulting with a SU supplier such as Sartorius at the facility design stage will result in better definition of space and layout requirements. In the long term, using a process-centric approach that is mindful of legacy equipment and fixed process needs can help prevent expensive mistakes in facility design and deliver the optimum layout in terms of cost, timelines, and safety.
References
1 Sartorius Bioprocess Development & Engineering Service; https://www.sartorius.com/en/services/bioprocess-development-engineering.
2 Sartorius Conceptual Design Digital Tool; https://www.sartorius.com/en/services/bioprocess-development-engineering/conceptual-design.
3 Adams B. RemeGen Grabs One of the World’s Largest Ever Biotech IPOs. FierceBiotech, November 2020; https://www.fiercebiotech.com/biotech/remegen-grabs-one-world-s-largest-ever-biotech-ipos.
4 Matica Biotechnology, Inc. Announces Groundbreaking for New GMP Facility. Matica Biotechnology press release, February 2021; https://www.maticabio.com/pr-groundbreaking.
Nihit Singhal is team lead for process consulting and a process layout design expert (process architect) at Sartorius AG in Germany; [email protected].
You May Also Like





