- Sponsored Content
Flavivirus Vaccine Production Accelerates with Modern Bioprocess Tools and SolutionsFlavivirus Vaccine Production Accelerates with Modern Bioprocess Tools and Solutions
November 22, 2017
Sponsored Content
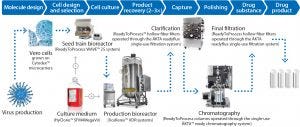
Figure 1: Process train comprises single-use equipment from GE Healthcare that help accelerate flavivirus vaccine manufacturing. Included systems are suitable for biomanufacturing of regulated products under various quality management systems. The systems are controlled through either GE Healthcare’s UNICORN™ or Schneider Electric’s Wonderware® system control software. To enable use of the systems in regulated environments, both programs are configured for use in a 21 CFR Part 11 and GAMP 5 compliant manner. All records are stored in a single, unalterable database, including results and extended run documentation. Specially trained and certified engineers perform onsite IQ/OQs and CCPs in accordance with cGMP, as well as provide on-site training for relevant personnel.
As with all viral vaccines, the complex nature of flaviviruses makes process development technically challenging. In addition, vaccine production can be both costly and difficult to scale to meet market demands. In egg-based vaccine production, for example, 100–300 vaccine doses can be produced from one fertilized hen’s egg. However, the eggs used for production need to be supplied from special pathogen-free chicken flocks, limiting availability of eggs and making vaccine production difficult to scale up. To meet the needs of preventive campaigns, including routine immunization and emergency response stockpiling, millions of vaccine doses would be required, making production both space- and resource-consuming.
For a more efficient response to market needs, cell-based vaccine production can be an alternative to egg-based production. However, cell-based vaccine production is traditionally performed in stainless steel bioreactors that require extensive cleaning and sterilization preparation time. Alternative single-use equipment minimizes the need for costly and time-consuming cleaning operations, as manufacturing components that have been in contact with the process material can be disposed of after use. Single-use equipment also minimizes cross-contamination risk and contributes to increased operator safety by eliminating the need for open handling of products. The reduced need for cleaning and cleaning validation allows for quick start-up and changeover between production campaigns. Because less cleanroom space is required, single-use technologies help reduce manufacturing footprint as well as costs for utilities, heating, ventilation, and air conditioning (Figure 1).
Cells commonly used for virus propagation, such as Vero cells, are anchorage dependent and can only proliferate when provided a suitable surface. To meet that need in bioreactor cultures, microcarriers are used. Compared with traditional shake-flask systems and roller-bottles, microcarriers provide a larger ratio of surface area to volume, enabling production of higher titers in a reduced footprint. Increasing upstream titers, however, puts pressure on capacity in downstream purification processes. Chromatography provides a highly selective and scalable alternative to purification techniques such as precipitation and ultracentrifugation. Compared with legacy products, modern chromatography resins offer improved pressure and flow properties that increase productivity. With such features, more product can be produced within a shorter period, making modern resins more suited for manufacturing applications than legacy products. In vaccine production, a short time to market is not only beneficial for the manufacturer, but for patients too.
Addressing Shear Sensitivity in Adherent Cultures
Adherent cells are sensitive to shear stress. A rocking bioreactor system provides gentle agitation of the culture to better control shear stress while providing sufficient aeration of the culture. Single-use rocking bioreactor systems are available for application such as process development, seed culturing, and small-scale productions. Although rocking systems have a different vessel geometry, studies have shown that they can give a representative reflection of processes performed in a stirred-tank bioreactor (1). Hence, rocking bioreactor systems also can be used as scale-down bioreactor from a stirred-tank system.

Figure 2: Designed for scalability and robustness, the Xcellerex XDR bioreactor system platform provides the performance and flexibility needed from process development to large-scale biopharmaceutical manufacturing. The complete range of XDR bioreactor systems are available with maximum working volumes ranging from 10 L to 2,000 L, from the smallest XDR-10 to the largest XDR-2000 system.
Single-use stirred-tank bioreactor and fermentor systems are based on the same principles as conventional stainless steel bioreactors. Traditional scaling methodology, based on measures such as shear, tip speed, power per unit volume, kLa, and specific process sensitivities, can be used during scale-up. With stirred-tank system platforms, technology transfer is straightforward, minimizing the need for costly and time-consuming process redesign (Figure 2).
Figure 3: Cytodex Gamma microcarriers are delivered gamma sterilized and ready for use for quick culture startup. In addition, Cytodex Gamma matrix provides a stable but nonrigid, tissue-like substrate for stirred cultures. Dextranbased microcarriers are translucent, allowing for easy microscopic examination of attached cells.
In bioreactor cultures, microcarriers are used to provide a suitable growth surface for the adherent cells commonly used in virus production (Figure 3). Microcarriers based on low-density dextran beads enable easy mixing and low shear (2). Bead size and density are optimized to support high cell growth rate and yield. The biologically inert polysaccharide products are supplied dry and shrunken to save storage space and facilitate transportation. To simplify transfer to cell culture vessels, the microcarrier container is equipped with flexible connection options.
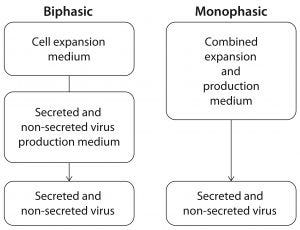
Figure 4: Whereas traditional virus production in EB66 cells is biphasic, requiring two or more media and multiple additives, CDM4Avian medium is designed to support the simpler monophasic approach, requiring fewer additives.
Whereas many cell lines employed in vaccine production are obligate attachment cells, the EB66® cell line (Valneva), derived from duck embryonic stem cells, grows in serum-free suspension culture at high cell density, allowing for easy and efficient scale-up (Figure 4). EB66 cells form loose aggregate structures that facilitate infection of nonsecreted, cell-to-cell transmitted viruses (3). To increase cell density and virus titer, both microcarrier-based adherent and suspension cell cultures can be run in perfusion mode using bioreactors equipped with cell-retention filters (4, 5).
Increasing Productivity in Upstream Operations
Selection of the right cell culture medium is important to enhance process yields in the manufacture of viral vaccines. For regulatory readiness, a cell culture medium free of animal-derived components is recommended. Modern culture media are developed to provide optimized conditions for high cell growth and productivity. However, the cell culture medium and feed strategy should be selected with respect to the nutritional requirements of the specific cell clone used.
Nutrient concentrations need to be kept within a certain range, as concentrations that too high or too low can be detrimental to the cells. Design of experiment (DoE) methodologies can be used to identify component groups in the medium that have the greatest effect on cell growth and productivity. This approach produces maximum amount of data with minimum number of experiments and meets the demands from regulatory authorities for better process understanding, one of the cornerstones of the quality by design (QbD) initiative.
Achieving Efficiency in Downstream Purification
DoE methodology also can be used for identifying parameters affecting purity and yield in downstream processes. Once the chromatography resins are selected, conditions for optimal hcDNA and HCP reduction at maximal product recovery are determined.

Figure 5: Schematic representation of Capto™ Core 700 shows a bead with the inactive, porous shell and the ligandcontaining core. Proteins and impurities penetrate the core, while target viruses and larger biomolecules (>Mr 700,000) are excluded from the resin and pass in the flowthrough.
Both cation-exchange and anion-exchange chromatography resins commonly are used to reduce impurity levels in virus vaccine purification processes. There are also examples of affinity chromatography resins with ligands that exhibit affinity for specific viruses such as the adenoassociated virus. For more challenging separations, multimodal resins with multiple modes of actions (ion exchange, hydrophobic interaction, and hydrogen bonding) can be used. In recent years, a new class of multimodal resins has been developed. In those resins, dual layers have been introduced in the bead design, combining size-exclusion properties from an inactive outer layer with adsorption chromatography from a ligand-activated core (Figure 5). Small molecules enter the core, where they are captured; viruses and other large entities are excluded and can be collected in the flowthrough.
Modern resins are designed for large-scale chromatographic processes, where high throughput and process economy are essential. Their base matrices have exceptional mechanical stability and optimized pore size to enable efficient capture under high-flow conditions. The improved mechanical stability also increases flexibility in terms of bed height and the ability to process highly viscous feeds. The chemical stability of these resins ensures a long lifetime even when harsh cleaning procedures are used. By offering a combination of high volume throughput and capacity, modern resins provide a powerful solution for fast and efficient processing of large amounts of protein. When high throughput is of utmost importance, membrane chromatography is an alternative option. Chromatography membranes exhibit a high porosity suitable for virus purification while providing the opportunity for using high flow rates.

Figure 6: GE Healthcare’s 750 C hollow-fiber filter, with a Mr 750,000 nominal molecular weight cutoff (NMWC), is designed for use in virus-purification workflows. It effectively removes ovalbumin and other proteins in allantoic fluid from egg-based virus production as well as host-cell–derived impurities from production in cells. When compared with a 500 C hollow-fiber filter — with the same 0.5-mm lumen diameter but a Mr 500,000 NMWC — in a concentration and diafiltration process, the more open structure of the 750 C filter gave a 1.5–2.0 orders of magnitude higher host cell DNA (hcDNA) removal at similar host cell protein (HCP) removal and virus yield (6).
Filtration of Delicate Targets: Cross-flow filtration (CFF), also known as tangential-flow filtration (TFF), is a technique extensively used in vaccine production. In contrast to normal flow filtration (NFF), the feed is recirculated over a permeable membrane surface. In CFF, liquid and compounds with molecular weights less than the membrane cut-off can pass through the membrane, whereas larger molecules or particulates are retained and concentrated. For delicate targets, such as the flavivirus, hollow-fiber filters are commonly used for the CFF step. Because of the open channel structure, a hollow-fiber filter usually causes less damage to the target product than does a filter cassette (Figure 6). For virus particles expressed in low titers that need to be concentrated as much as 200× to 500× before further processing, single-use tubing assemblies can be used in the design of circuits with low working volumes to enable high concentration factors (7).
Gaining Insights with Versatile Analysis Technology
The complex nature of viruses also presents challenges for process analytics. Ideally, analytical methods for vaccine characterization are developed in parallel with process development to aid in gaining regulatory approval and for further manufacturing.
Vaccine design depends on structural and functional interactions with the host immune system. Label-free molecular interaction analysis based on surface plasmon resonance (SPR) is extensively used in vaccine development and production in areas such as design and characterization, immune response studies, vaccine quantitation, and in analyses during production and quality control. As has been shown with Zika virus, for example, interaction data can be used to gain insights into the binding of neutralizing antibodies to viral epitopes (8). Using SPR, detailed information also can be obtained from analyses of binding kinetics, specificity, immune responses, epitope mapping, and concentration (9).
Case Study on Improving Purity of Flavivirus
To meet the concerns with live, attenuated vaccines, a client process for production of inactivated whole-virus yellow fever vaccine was developed by GE’s Fast Trak Services team (10). Virus was produced in adherent Vero cells grown on Cytodex 1 microcarriers in medium that was free of animal-derived components and supplemented with recombinant human albumin, using the XDR-50 bioreactor system. The system was selected because it features many properties that address the requirements of the shear-sensitive culture. The impeller is designed with an optimized profile, angle, and number of impeller blades to provide good mixing while minimizing shear forces. A broad and adjustable agitation speed range also supports a well-mixed tank without undue shear effects on the cells. The gas-sparging discs provide both micro- and macrosparging capabilities for effective mass transfer. The proximity of the gas-sparging discs relative to the impeller shear zone ensures excellent gas dispersion, especially at low gas flow rates.
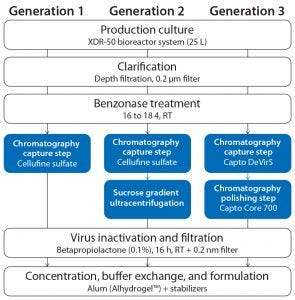
Figure 7: The initial virus production process, including purification on Cellufine sulfate resin, was complemented with an ultrafiltration step to improve HCP reduction. In the final optimized process, Cellufine sulfate and ultracentrifugation steps were replaced by two chromatography steps based on Capto resins.
Downstream purification was optimized as summarized in Figure 7. Initially, the purification process was based on affinity chromatography using Cellufine™ sulfate resin (Chisso Corp.) (Generation 1). Because that purification approach resulted in insufficient HCP removal, a sucrose-gradient ultracentrifugation step was included to increase purity of the virus (Generation 2). However, that step was found to be cumbersome and costly, and not all manufacturing facilities have access to the required equipment. Therefore, the purification process was further optimized by replacing it with two simple chromatography steps (Generation 3). Capto DeVirS exhibits an affinity-like behavior for several virus types and was selected for the initial capture step. Capto Core 700 was chosen for its efficient removal of remaining impurities in the subsequent polishing step.
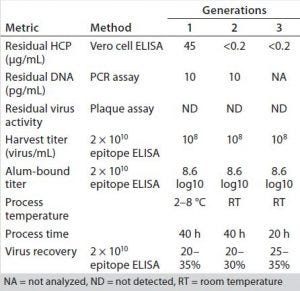
Table 1: Results from the optimized process compared with those from the initially developed process
Compared with the legacy process (Generation 1), HCP was significantly reduced at a similar or even slightly higher recovery in half the process time (Table 1). With the optimized downstream purification process, manufacturability was significantly approved. In addition to removing the ultracentrifugation step, the optimized process could be conducted at room temperature (RT), in contrast to the legacy process performed at 2–8 °C. The developed process is easily scaled and compatible with both single-use and conventional technologies, and all process materials meet stringent regulatory requirements.
Conclusion
Technological challenges can dominate vaccine production. This case study gives an overview of modern products and services that can help solve many challenges in flavivirus vaccine production. Bioreactor systems based on single-use technologies support significant time savings while increasing process and operator safety in cell-based vaccine production. Microcarriers provide the cell surface required for high volumetric productivity of adherent cells in bioreactor cultures. With modern chromatography resins, more product can be purified within a given time frame.
Label-free molecular interaction analysis based on SPR technology, can be used for reliable quantification and characterization of the end product. Regulatory-friendly system-control software allows equipment to be used in a cGMP-compliant manner. Modern vaccine production platforms support reduced process time and cost to help accelerate your flavivirus vaccine production.
References
1 29119376, Edition AA. Application Note: Efficient, High-Titer Monoclonal Antibody Production in a Fed-Batch Process Using Single-Use Stirred-Tank and Rocking Bioreactor Systems. GE Healthcare: Uppsala, Sweden, 2014.
2 29209415, Edition AA. Application Note: Validation of the Production of Influenza Virus in ReadyToProcess WAVE 25 Bioreactor System, Comparing Cytodex and Cytodex Gamma Microcarriers. GE Healthcare: Uppsala, Sweden, 2016.
3 29235842, Edition AA. Application Note: Development of a Chemically Defined Medium for Virus Production in Suspension-Adapted Avian Cells. GE Healthcare: Uppsala, Sweden, 2017.
4 Nikolay A, et al. Evaluation of Producer Cell Lines in Yellow Fever Virus Production in Up to 1-L Bioreactor Scale. Poster at Vaccine Technology VI, 12–17 June 2016.
5 Nikolay A, et al. Propagation of Brazilian Zika Virus Strains in Static, Microcarrier-Based and Suspension Cultures Using BHK and Vero Cells. Poster at Vaccine Technology VI, 12–17 June 2016.
6 29092826, Edition AA. Application Note: Concentration and Diafiltration of Cell-Derived, Live Influenza Virus Using 750 C Hollow-Fiber Filter Cartridge. GE Healthcare: Uppsala, Sweden, 2014.
7 29228378, Edition AA. Application Note: Efficient Concentration of a Low-Titer Bovine IgG with High Recovery in Low Volume. GE Healthcare: Uppsala, Sweden, 2016.
8 Dai L, et al. Structures of the Zika Virus Envelope Protein and Its Complex with a Flavivirus Broadly Protective Antibody. Cell Host & Microbe 19, 2016: 696–704.
9 28987028, Edition AB. White Paper: Biacore Systems in Vaccine Development and Production. GE Healthcare: Uppsala, Sweden, 2016.
10 Monath, et al. Inactivated Yellow Fever 17D Vaccine: Development and Nonclinical Safety, Immunogenicity and Protective Activity. Vaccine 28, 2010: 3827–3840.
You May Also Like





