- Sponsored Content
- Emerging Therapeutics
- Separation/Purification
Intensification of Fab-Fragment Purification: Multicolumn Chromatography Using Prepacked Protein L ColumnsIntensification of Fab-Fragment Purification: Multicolumn Chromatography Using Prepacked Protein L Columns
Sponsored by Tosoh Bioscience

Photo 1: Octave BIO system.
Antibody fragments — such as fragment antigen-binding (Fab) domains, single-chain variable fragments (ScFvs), and heavy-chain variable domains (nanobodies) — have emerged as increasingly important therapeutic and diagnostic alternatives to full-length monoclonal antibodies (MAbs) for a multitude of diseases. Whereas MAb downstream processing is well established and easy to scale based on protein A capture, the purification of antibody fragments is just on the verge of standardized processing. The most promising candidate for effective capture of those containing a kappa light chain is protein L affinity chromatography in the first step of downstream processing (1).
To establish an effective capture process for antibody-fragment purification, we compared the capabilities of two different protein L chromatography resins in batch mode and then investigated their performance using multicolumn chromatography (MCC) to enhance process performance further. Combining a resin with high loading capacity and MCC technology provided high resin and buffer savings while lowering process operation costs for manufacture of clinical materials.
Antibody Fragments and Resins
Tosoh Bioscience’s Toyopearl AF r-protein L-650F resin is based on polymethacrylate particles. It offers a static binding capacity (SBC) of 54 mg/mL for Fab fragments and remains stable at pH 2–12. For comparison, we also performed all experiments with Capto L resin from Cytiva.
To prepare an antibody-fragment feedstock solution, MAb purified by protein A was mixed with activated papain, which cleaved the molecules after the histidine residue (His-228) in a hinge region to separate their Fab fragments individually from the fragment crystallizable (Fc) regions. The reaction mixture was spiked with cell-culture supernatant from Chinese hamster ovary (CHO) cells to simulate a feedstock with a final Fab fragment concentration of 2.23 mg/mL containing impurities that typically are present before the capture step.
Batch Purification: After method optimization, we applied the following conditions for batch chromatography with both resins using 2.4-mL columns with 7-cm bed heights:
• Equilibration — 5 column volumes (CV) of 100 mM sodium phosphate, pH 7.0
• Load — variable CV of feed containing Fab fragments
• Wash 1 — 10 CV of 100 mM sodium phosphate, pH 7.0
• Wash 2 — 5 CV of 100 mM sodium acetate,
pH 5.5
• Elution — 5 CV of 100 mM glycine with hydrochloric acid (HCl), pH 2.5
• Clean in place (CIP) — 5 CV of 50 mM sodium hydroxide
• Reequilibration — 5 CV of 100 mM sodium phosphate, pH 7.0.
Columns were loaded with 85% of their previously determined dynamic binding capacity (DBC) to ensure that no product is lost during the loading step. This resulted in a loading volume of 15 CV (33.5 mg/mL resin) for the Tosoh resin and 6 CV (13.4 mg/mL resin) for the comparator. The elution peak was collected and analyzed using size-exclusion ultrahigh-pressure liquid chromatography (UHPLC) for the impurity profile and analytical protein L chromatography for concentration. We calculated product recovery from the results of those analyses.
Continuous Purification: We translated the above purification method to an MCC process using Tosoh Bioscience’s ProComposer Method Wizard tool, with a goal of improving process productivity while decreasing buffer consumption. To achieve that goal, an Octave BIO system (Photo 1) was equipped with five 1-mL SkillPak BIO prepacked columns (all from Tosoh Bioscience) containing the respective protein L resins.
The Octave BIO system consists of six pumps, a switching-valve block, and a detector array. Each pump is designated for one buffer required in the process. Through the valve block, up to eight columns can be addressed by different pumps in parallel or connected to each other in series. The detector array provides precise control of up to four different process streams with regard to UV absorption, conductivity, and pH.
For the MCC process, we connected three of the five columns in series through the valve block. That enabled increased loading amounts and resin-capacity use by catching product breakthrough on subsequent columns. While those three columns are in the loading phase, the remaining two go through the phases of wash and elute, then CIP and equilibration. Once the first column in the loading series is fully loaded, the column ports are switched into position against the flow stream. Similar configurations are proven to be most effective for capture processes (2). Each MCC experiment consisted of five cycles in which columns rotated through the different process steps.
In MCC, product breakthrough is less of a risk during loading because of the secondary load columns. Thus, we loaded the columns to 85% of their previously determined SBCs, resulting in loading masses of 45.3 mg/mL resin for the Tosoh resin and 21.62 mg/mL resin for the comparator. Both went through two runs, with residence times during loading varying from 0.5 minutes to 0.25 minutes.
Intensified Protein L Capture
We compared the results of these experiments based on several key parameters (Table 1). Clearly, using three load columns on the Octave BIO system proved effective for maintaining high recovery while increasing the capacity use of the resins over that of batch processes. When comparing MCC results with different residence times, we found 0.5 minutes to be favorable for high recovery — a value we also had found in previous studies of protein A capture (2). However, for processes focused on productivity, 0.25-min residence time would speed up a run while maintaining recovery at acceptable levels.
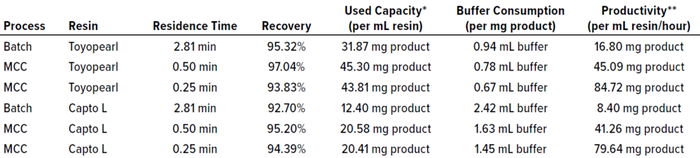
Table 1: Comparing evaluation parameters for different Fab purification runs.
* Expresses how much product can be recovered per run with respect to the resin bed volume.
** Expresses process efficiency measured in terms of the mass of product processed per unit volume of resin over time.
Maximizing resin-capacity use positively influences process economy by increasing the mass of protein that can be purified per liter of purchased resin. In MCC, the first column in the loading phase can be loaded with more protein because the subsequent columns will catch breakthrough product that might break through. Thus, capacity use can be improved by 42% for the Tosoh resin and by 66% for the comparator. Our study revealed that about 2.2× more protein mass can be loaded onto the Tosoh resin than onto the Cytiva resin, which can be attributed to their different SBCs.
Comparing the buffer consumption of batch and multicolumn chromatographic methods, it is clear that MCC contributes significantly to reducing chemical consumption and/or waste generation through improved resin use. Such an improvement in process economy can be enhanced by using a resin with higher binding capacity, as illustrated by the comparison herein.
Productivity depends mostly on process duration. Reducing the residence time during column loading directly increases productivity by nearly the same factor. Compared with the batch runs, MCC increases productivity by roughly 170% through shortened load residence time (to 0.5 minutes) — and by another 235% through shortened residence time (0.25 minutes). The reduction in process duration is reflected in operating costs because productivity and process efficiency are proportional to resin and time savings.
Our comparison highlights the benefit of converting the antibody-fragment capture step to a continuous process. The higher capacity use, increased productivity, and reduced buffer consumption demonstrated herein all improve process economics and increase the feasibility of purifying large quantities of Fab-containing fragments in a reduced time. The Octave BIO system facilitates such a transition from batch to continuous processing both through its intuitive handling and by simplifying method transfer.
References
1 Bumelis VA, Markmann A. High Value Biopharma Process Development. Euro. Biotechnol. 22, Spring 2023: 54–55; https://european-biotechnology.com/up-to-date/backgrounds-stories/story/high-value-biopharma-process-development.html.
2 Mabry J, et al. Two-Step Monoclonal Antibody Purification Using a Multicolumn Continuous Chromatography Platform. BioProcess Int. 20(10) 2022: 54–56; https://bioprocessintl.com/sponsored-content/two-step-monoclonal-antibody-purification-using-a-multicolumn-continuous-chromatography-platform.
Corresponding author Sebastian Thürmann is product manager for multicolumn chromatography at Tosoh Bioscience GmbH, Leuschnerpark 4, 64347 Griesheim, Germany; 49-6155-7043700; [email protected]. Patrick Endres is EMEA product manager, Jonas Wege is an application-development manager, and Egbert Müller is a technical director, all at Tosoh Bioscience.
Toyopearl and Tosoh Bioscience are registered trademarks of Tosoh Corporation. Octave is a trademark of Tosoh Bioscience Wisconsin, Inc. SkillPak is a trademark of Tosoh Bioscience LLC. Capto is a registered trademark of Cytiva.
You May Also Like



.jpg?width=700&auto=webp&quality=80&disable=upscale)

