- Sponsored Content
- Chromatography
- MAb
- Separation/Purification
Two-Step Monoclonal Antibody Purification Using a Multicolumn Continuous Chromatography PlatformTwo-Step Monoclonal Antibody Purification Using a Multicolumn Continuous Chromatography Platform
Sponsored by Tosoh Bioscience

Photo 1: Bench-top Octave BIO MCC instrument with SkillPak columns.
Biomanufacturers typically have relied on multistep processes for optimal removal of impurities such as host-cell proteins (HCPs), DNA, adventitious viruses, and aggregates. However, additional purification steps increase downstream expenses significantly, including costs of supplementary resin, hardware, and buffers. The substantial footprint required at a processing site and additional time needed to perform a complete multistep purification process also increase production costs and complicate process execution. Thus, it is imperative to design and test effective purification procedures for high-quality biotherapeutics, but reasonable process costs, time, and manufacturing space are required.
Batch purification platforms for monoclonal antibodies (MAbs) typically contain an affinity capture step based on protein A followed by polishing steps for further impurities reduction. Innovations in downstream MAb processing, including multicolumn continuous chromatography (MCC) instrumentation, have been shown to increase MAb purification productivity and reduce operating costs significantly. Although batch processes are inherently less complex, MCC takes significant advantage of spreading the sample load across multiple columns in a cyclical process. As a result, a several-fold increase in productivity typically is achieved because of resin, buffer, and time savings (1, 2).
To demonstrate the benefits of MCC technology in downstream processing, we describe a two-step MCC platform for MAb purification using Tosoh Bioscience’s Octave BIO benchtop instrument (Photo 1) and SkillPak BIO prepacked columns optimized for MCC applications.
MCC Instrumentation and Chromatography Resins
The Octave BIO bench-top MCC system is based on rugged, reliable, and patented Octave technology made for bioseparation. The system can run methods using up to eight columns using six pumps. It contains a low–dead-volume replaceable manifold block (valve block), which is hydraulically actuated to regulate flow within each column. Three exchangeable valve-block sizes account for four different sizes of pumps with flow rates ranging from 12 to 300 mL/min. For complete data recording, the system has integrated detectors (four of each) for UV, conductivity, and pH. The system can run gradients in single-column batch mode with a sample injector. That is particularly useful for initial resin and method development. Furthermore, a three-way peak-collect valve enables collection of highly concentrated product.
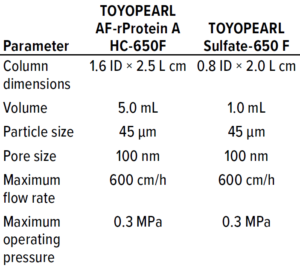
Table 1: Specifications of SkillPak BIO columns for multicolumn continuous chromatography.
TOYOPEARL AF-rProtein A HC-650F high-capacity affinity resin can be used for MAb capture. Protein A exhibits a competitive binding profile, especially at fast flow rates, which is a highly sought attribute for MCC processes. The maximal binding capacity of ~70 g MAb/L resin typically is observed for this resin in MCC operations. TOYOPEARL Sulfate-650F resin is a strong cation-exchange resin that provides separation of MAb aggregates. It has a high salt tolerance, a wide working pH range, and a dynamic binding capacity of >120 g MAb/L. The resin is packed into Tosoh Bioscience’s proprietary SkillPak BIO columns (Table 1), which are specifically suited for the Octave BIO system.

Table 2: Protein A process parameters for multicolumn continuous chromatography.
Capture Step on a Protein A Resin
The protein A–based capture step is well established in batch mode, in which MAb-binding and postloading wash are carried out at neutral pH followed by low-pH MAb elution. In the study herein, a similar purification process was tested in MCC mode (Table 2) using the Octave BIO system. Protein A columns (5 mL) were equilibrated and loaded with MAb-containing clarified Chinese hamster ovary (CHO) cell culture supernatant (titer 6.5 g/L) to ~90% of column capacity. Loading was optimized onto three columns in series with an additional two columns performing the remaining wash, elution, and CIP steps (Figure 1). Elution was carried out at pH 3.0 with the AU280-nm analysis measuring >200 mAU redirected through a three-way peak-collect valve and pooled separately from the rest of the process elution stream.
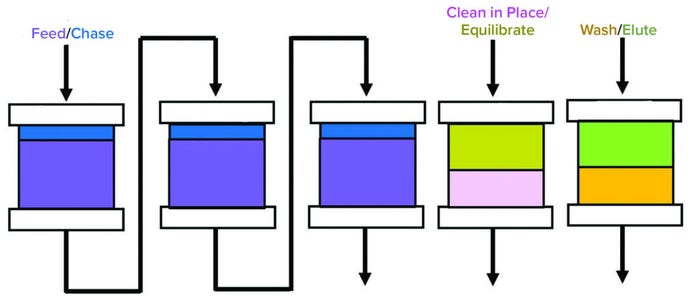
Figure 1: Multicolumn continuous chromatography protein A method; three columns in
series are in loading. Once the first column is completely saturated, it is moved to the
washing state, and the second column that was capturing the breakthrough moves into
the primary capture position. Subsequently, the columns are washed, and MAbcontaining
fraction is eluted through a separate outlet. The columns are cleaned and
reequilibrated before reentering the load zone.
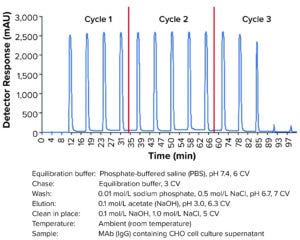
Figure 2: Multicolumn chromatography protein A elution chromatogram; AU280 trace of the elution outlet during run is in blue. Cycle changes are indicated as red lines. Cycle 1 represents the start-up mode, in which the first elution at ~6 min is without protein, and cycle 3 is the shut-down mode, in which the two last elution steps are directed to columns with no loaded protein.
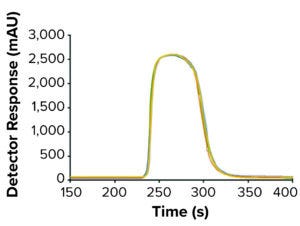
Figure 3: Protein A column-to-column variation during cycle 2; the different colors represent the five columns used in multicolumn continuous chromatography mode. An overlapped image of the UV280 elution peaks from each column is shown.
The MCC method was tested with three purification cycles in five-column mode. Figure 2 shows the resulting elution chromatograph exhibiting reproducible elution peaks in a saw-tooth pattern with a negligible column-to-column variation during cycle 2 in an overlapped image (Figure 3). The yield (92.1%), concentration of the main elution fraction (19.3 mg/mL), and mass balance in different fractions were as expected (Table 3).
For virus inactivation, the MAb-containing eluate was held at pH 3.8 for one hour before pH adjustment to 5.0 with 2 mol/L tris base. Because the capture step provided satisfactory purification and recovery of MAb, the next step was to find a suitable MCC polishing step, ideally benefiting from bind-and-elute chromatography mode, to enhance aggregate and HCP removal.
Polishing Step on a Sulfate Resin
TOYOPEARL Sulfate-650F resin was chosen for the polishing step because of its favorable pressure-flow characteristics and excellent impurity clearance in MAb processes. First, optimal NaCl concentration was tested for efficient elution of MAb from sulfate resin. A single-column linear salt gradient experiment at pH 5.0 using the Octave BIO system revealed the approximate conductivity (~41 mS/cm) needed for MAb elution (data not shown). Based on those results, 375 mmol/L NaCl in equilibration buffer was selected for the step elution.
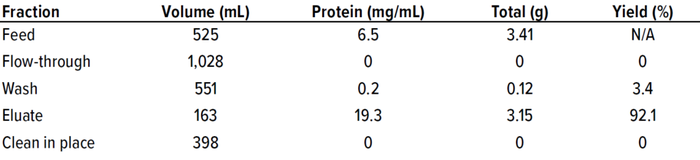
Table 3: Multicolumn chromatography protein A mass balance results.
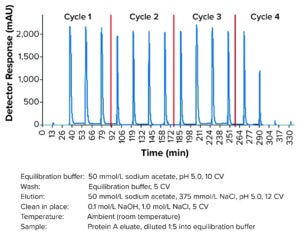
Figure 4: Multicolumn continuous chromatography Sulfate-650F elution chromatogram; AU280 trace of the elution outlet during run is in blue. Cycle changes are indicated as red lines. Cycle 1 represents the start-up, and cycle 4 is the shut-down mode.
Elution conditions from the single-column salt gradient elution were translated into a four-column Sulfate-650F MCC method. MAb loading was optimized over two columns in series to prevent significant MAb loss in the flow-through fraction while still maintaining high productivity. Two additional columns were required to complete the remaining wash, elution, CIP, and reequilibration steps. For eluate collection, the AU280-nm peaks measuring >200 mAU were pooled. The following MCC method in four cycles resulted in adequate elution profiles, including highly reproducible peaks (Figure 4) and an excellent yield (95.1%).
In summary, the final two-step process yield was 87% based on a 92.1% recovery from protein A and a 95.1% recovery from Sulfate-650F resin. Steady-state productivity for the protein A step was 106.4 g MAb/L resin/h, and the productivity value was 59.9 g MAb/L resin/h when the Sulfate-650F resin was used. For both MCC steps using the Octave BIO system, the productivity values thus were several-fold higher than those typically obtained from typical batch purification processes. Excellent HCP clearance (feed of 20,389 ng/mg protein; protein A eluate 302 ng/mg protein; Sulfate-650F eluate 29 ng/mg protein) and aggregate reduction (4.9% in protein A eluate; 1.2% in Sulfate-650F eluate) were obtained after each step.
Beneficial Two-Step Process
Herein we present a two-step MCC platform using an Octave BIO system with TOYOPEARL AF-rProtein-A HC-650F and Sulfate-650F resins. Results show highly satisfactory overall MAb recovery, purity, and process productivity. Using MCC helps reduce overall protein A requirements of ≤90% and reduce buffer consumption of 40–45%. The platform is straightforward to run and easily scalable to an industrial large-scale process using an Octave PRO skid (from Tosoh Bioscience LLC), which is built with the same technology. The Octave chromatography platform supports transformation of biologics purification toward MCC instrumentation because of the technology’s beneficial effects on product quality, time savings, and reduction of production costs.
References
1 Khanal O, Lenhoff AM. Developments and Opportunities in Continuous Biopharmaceutical Manufacturing. mAbs 13(1) 2021: 1903664; https://doi.org/10.1080/19420862.2021.1903664.
2 Matte A. Recent Advances and Future Directions in Downstream Processing of Therapeutic Antibodies. Int. J. Mol. Sci. 23(15) 2022: 8663; https://doi.org/ 10.3390/ijms23158663.
Jaclyn Mabry is applications scientist, Noah Makowicz is an intern from the University of Pittsburgh. Abbie Hevner is applications scientist, Sebastian Thürmann is product manager — MCC, Patrick Endres is manager — product management EMEA, Jonas Wege is application specialist, Mark Pagkaliwangan is global product manager — MCC, Kevin O’Donnell is R&D manager, Egbert Müller is director — scientific and applications development, and corresponding author Jukka Kervinen is manager, applications group; Tosoh Bioscience LLC, 3604 Horizon Drive, King of Prussia, PA 19406, USA; Tosoh Bioscience GmbH, Darmstadt, Germany; [email protected].
You May Also Like





