- Sponsored Content
Labfors 5 with LabCIP: Innovative Solution for High-Throughput Microbial CultureLabfors 5 with LabCIP: Innovative Solution for High-Throughput Microbial Culture
August 11, 2016
Sponsored by Infors-HT
 The need for a rapid turn-around in quantity (and the loss of technicians in many laboratories) has created a trend toward implementation of single-use vessels. Their advantages include no time lost in cleaning or sterilization (especially for bench-scale, autoclavable systems), no chance of inhibition or cross-contamination due to poor cleaning, and no need for cleaning and sterilization facilities in a crowded laboratory.
The need for a rapid turn-around in quantity (and the loss of technicians in many laboratories) has created a trend toward implementation of single-use vessels. Their advantages include no time lost in cleaning or sterilization (especially for bench-scale, autoclavable systems), no chance of inhibition or cross-contamination due to poor cleaning, and no need for cleaning and sterilization facilities in a crowded laboratory.
For production-scale steel bioreactors, fully automated clean-in-place (CIP) and sterilization-in-place (SIP) have been possible for decades. By contrast, bench-scale systems required considerable downtime for disassembly, cleaning, reassembly, autoclaving, and cooling before use — with no guarantee that each step was carried out correctly and reproducibly. An innovation from INFORS HT brings the advantages of CIP/SIP to bench-scale bioreactors, offering a true alternative to single-use systems for microbial applications.
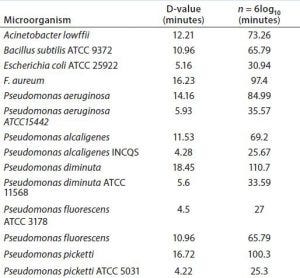
Table 1: Sterility testing against a panel of highly resistant test organisms
The LabCIP system provides user-configurable recipes for cleaning, sterilization, and rinsing processes. It can be left to perform those tasks overnight so that vessels are ready to use the next day. For high-throughput fermentation with organisms such as Escherichia coli, that capability can double the productivity of each bioreactor. The system is reliable and reproducible, and it involves little input beyond priming reagent bottles and pressing “go.” Despite all this functionality, the LabCIP module’s footprint is smaller than an A4 sheet of paper.
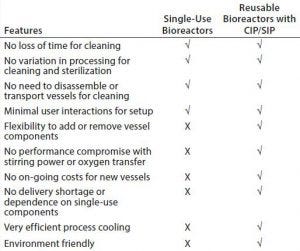
Table 2: Comparison of single-use systems and self-cleaning and self-sterilizing bioreactor with the LabCIP system
The effectiveness of sterilization with sodium hydroxide is high, even at a low temperature of 25 °C (Table 1). A typical sterilization procedure using a LabCIP system is carried out at 60 °C, which speeds up the sterilization process dramatically. Sterility testing against a panel of test organisms (E. coli, Pichia pastoris, and highly resistant Geobacillus stearothermophilus spores) showed no contamination after six days of incubation following the CIP/SIP process.
Direct comparison of single-use systems and a self-cleaning, self-sterilizing bioreactor with LabCIP reveals the advantages of the new system (Table 2). Advances in automation for cleaning and sterilization now provide the best of all possibilities: customizable, high-performance bioreactors with no downtime related to manual cleaning and sterilization.
References
1 Mazzola PG, Martins AMS, Penna TCV. Chemical Resistance of the Gram-Negative Bacteria to Different Sanitizers in a Water Purification System. BMC Infectious Diseases 6, 2006: 131.
Dr. Tony Allman is product manager fermentation at INFORS HT, Rittergasse 27, CH-4103 Bottmingen/Basel, Switzerland, 41-61-425-7700, fax 41-61-425-7701; [email protected], www.infors-ht.com.
You May Also Like






