- Sponsored Content
- Single Use
Perspectives on Bioseparation and Single-Use CentrifugationPerspectives on Bioseparation and Single-Use Centrifugation
August 22, 2022
Sponsored by Rau Consulting
Although single-use technologies have been part of downstream processing for at least 20 years, single-use centrifugation systems have been developed only in the past decade. They offer significant advantages over traditional centrifugation methods, and suppliers are developing single-use centrifuge lines for shear-sensitive applications. To enrich our understanding of the past 20 years of bioprocessing, BPI distributed questions to supplier companies. Below, Tiffany Rau (owner and principal consultant of Rau Consulting) provides her perspective on the breakthroughs in bioseparation and the advantages that can be gained from using a unique line of single-use centrifugation systems.
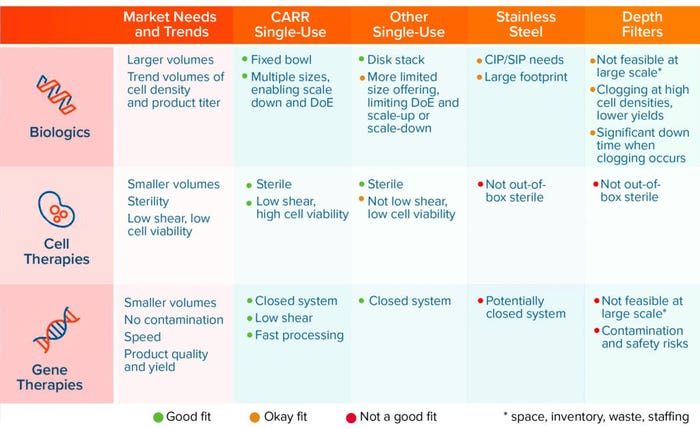
Figure 1: Single-use versatility addressing multiple markets; important attributes of different technologies (CIP = clean in place, SIP = sterilize in place)
Breakthroughs in Bioseparation Systems
Until a decade ago, biomanufacturers had limited options for performing bioseparations. Technologies included bench-top, floor, fixed-bowl, and disk-stack stainless-steel centrifuges and filtration technologies such as depth filters and filter presses. Although those systems worked well for many bioprocesses, they were not well suited for processes that had to be sterile from start to finish (e.g., processes for cell therapies). Requirements for cleaning, utilities, and space restricted the use of such technologies, especially in small facilities and in contract development and manufacturing organizations (CDMOs) that demanded quick changeovers.
Biomanufacturers working with advanced biologics (e.g., cell therapies) needed new solutions and tried using tools designed for other purposes such as blood processing. Those solutions were ideal for personalized, small-volume therapies, but not for large volumes required for allogeneic cell therapies or monoclonal antibodies. Medical device regulations for blood products also are different from those that apply to biologics. Emerging therapy processes and technologies needed to mature to meet the requirements of applications requiring good manufacturing practice (GMP).
In the past decade, single-use centrifuges (e.g., UniFuge centrifuges from CARR Biosystems) have been developed. They meet process requirements for late-stage or commercial therapies, including GMP expectations with 21 CFR 2010/211 compliance. Single-use centrifuges are reliable alternatives to other separation instruments such as stainless-steel centrifuges and depth filters. They also can be used complementarily or separately to deliver a robust unit operation. Thus, single-use centrifuges have become permanent parts of bioprocessing.
Benefits of Implementing Single-Use Technologies
Every segment of the biopharmaceutical industry can benefit from using single-use technologies, especially in processes that require strict contamination and safety controls. Single-use centrifuges have been used in cell and gene therapies, vaccines, large molecules, mRNA, cellular agriculture, and even industrial biotechnology products. The UniFuge line of single-use centrifuges, for example, is offered in three sizes to suit the needs of multiple applications and to allow for seamless scale-up, scale-down, and scale-out. Traditionally, processes that have been >2,000 L combined stainless-steel centrifugation with filtration. Now, processes that complement depth filtration and single-use centrifugation are common. Depth filters polish material streams coming out of single-use centrifuges before proceeding to the next unit operation, which for large molecules is typically a chromatography column.
Some products that cannot be terminally sterilized (e.g., cell and gene therapies) require closed-system, single-use technologies because processes must run aseptically from start to finish. Single-use centrifuges enable closed-system processing of cell therapies during separation and washing processes, thereby minimizing manual operations, reducing contamination risk, and decreasing the level of process variability. Biomanufacturers of cell and gene therapies also need vendors that take a collaborative approach on the science and ensure that equipment and consumables meet expectations from research and development to commercial GMP production.
Advantages of Using Single-Use Centrifuges
The key advantages of implementing single-use centrifuges can be organized into the following categories: performance, operations, process, environment, and health and safety.
Performance: Single-use centrifuges process material faster than depth-filters and address the risk of clogging posed by high-density or “sticky” cultures.
Operations: Single-use centrifuges require minimal infrastructure compared with stainless-steel centrifuges, and the footprint of single-use centrifuges is smaller than that required by depth filters. Single-use systems have one consumable, but depth filtration systems have many consumables.
Process: Single-use centrifuges have modules that are presterilized before they are sent to users. Single-use centrifuges are sent with all appropriate certifications for implementation into GMP manufacturing, such as those assuring that materials are free from transmissible spongiform encephalopathy (TSE) and bovine spongiform encephalopathy (BSE) and documentation of sterility and leachables information. Thus, no change of technology is necessary to move from R&D to manufacturing. Depth filters also have that documentation, but they do not provide a sterile unit operation.
Environment: Compared with other bioseparation methods, single-use centrifuges minimize waste because the number of filters used in primary recovery of a product is reduced or eliminated. Single-use systems do not require steam-in-place or cleaning protocols that require high energy consumption and often use harsh chemicals.
Safety: Single-use centrifuges enable closed processing, so bioseparations can be conducted in rooms with classifications that are lower than those for aseptic environments. Closed processing also ensures that products are free from “outside” contamination and that room environments are free from process contaminants.
The UniFuge line consists of state-of-the art, single-use centrifuges for shear-sensitive applications, enabling high-quality products and high product recovery rates — two key goals in biomanufacturing. UniFuge systems streamline bioprocesses, enabling rapid and smooth transitions from development laboratories to manufacturing suites as the next generation of therapies advances from clinical trials to patient administration.
Scientists must have the tools to develop processes from small to large scales. Historically, centrifugation caused problems during scale-up and even led to process delays in some cases because reliable scale-down models were not available. So process teams had to conduct experiments at scale rather than in a development laboratory. CARR Biosystems offers UF-Mini small-scale centrifuges that enable teams to design and derisk centrifugation steps early and prepare for formal process characterization and manufacturing support. Data collected in process development can support scale-up and process characterization work required for late-stage products. Having multiple sizes of equipment enables you to conduct a design of experiments early and to explore parameters such as flow rates, processing time, and temperature. Designing for manufacturability before reaching a manufacturing suite is critical for shortening time to market, improving cost of goods, and minimizing surprises. Having solutions that scale is only part of the equation for success. Technologies must be robust and be a good fit for feedstreams. For a cell therapy application, for example, cells must be separated from growth media, washed, and “formulated” while operators maintain high product quality. Conducting activities in a single unit operation with a minimum number of interactions increases efficiency and minimizes processing times, without “stop” and “go” steps. Single-use technologies also facilitate the use of equipment for several programs (e.g., for multiple products) without concerns of cross-contamination between programs and without the need of biosafety cabinets.
Cell Separation in the Long Term
Cell separation in future bioprocessing will leverage a range of technologies that address the needs of different processes, without the need of a “one-size-fits-all” solution. Single-use centrifuges will be key to biologics production and manufacturing and will become more of the norm instead of the exception.
Single-use centrifuges also will change the way the biopharmaceutical industry thinks about implementing centrifugation technologies around the world and how to develop products within smaller footprints and without the need for specific utilities. I also imagine a future industry with more partnering among biopharmaceutical companies and vendors to accelerate the development of tools that scientists and engineers need when new types of cells and products start to move through development and chemistry, manufacturing, and controls pipelines. We want to move away from situations in which processes and equipment are not GMP ready.
Tiffany D. Rau, PhD is the owner and principal consultant at Rau Consulting; [email protected]; https://carrbiosystems.com.
You May Also Like





