- Sponsored Content
Prescriptive Analytics for Bioprocessing PlatformsPrescriptive Analytics for Bioprocessing Platforms
December 11, 2017
Sponsored Content
 The preceding articles have shown how biopharmaceutical companies increasingly are adopting single-use manufacturing technologies for commercial production facilities as their confidence grows in the material science, supply chains, and robustness of single-use systems. Single-use suppliers can support adoption of end-to-end process platforms in commercial manufacturing settings with dedicated teams of experts in process development, engineering, and regulatory support. Advances in process analytical technology (PAT) are providing engineers with greater information on conditions within their single-use bioprocess platforms to allow for increasing levels of control.
The preceding articles have shown how biopharmaceutical companies increasingly are adopting single-use manufacturing technologies for commercial production facilities as their confidence grows in the material science, supply chains, and robustness of single-use systems. Single-use suppliers can support adoption of end-to-end process platforms in commercial manufacturing settings with dedicated teams of experts in process development, engineering, and regulatory support. Advances in process analytical technology (PAT) are providing engineers with greater information on conditions within their single-use bioprocess platforms to allow for increasing levels of control.
Engineers can define set points for important parameters such as pH, temperature, and dissolved oxygen to provide consistent operation, use PAT tools to measure necessary parameters such as glucose and cell density, and then use regulatory controllers to maintain those parameters at the predefined set points. Some problems come with using this approach in isolation. First, each parameter is controlled independently from the others. Controlling to set points does not necessarily ensure optimum quality or productivity outcomes, nor is the control mechanism updated with new information process variability. More sophisticated control strategies could bring more productive processes and more consistent product quality. The biomanufacturing sector needs the ability, not just to understand what is happening in a bioprocess (triggering an action), but also to understand what will happen in the future based on current conditions. If needed, interventions can be made to modify this future state.
Model predictive control (MPC) is a supervisory control method used to determine optimal set points for regulatory controllers described above for optimizing qualities and yields. For example, a regulatory controller can be used to adjust nutrient flow to maintain glucose at a desired set point, and an MPC controller can be used to determine the optimal glucose set point to maximize cell productivity or final titer. As the name implies, MPC requires a process model relating the influence of regulatory control set point adjustments to cell productivity (or any other control objective).
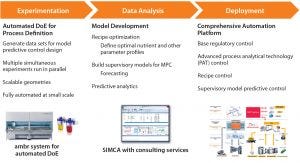
Figure 1: Stepwise implementation of model predictive control for a cell culture process
As Figure 1 illustrates, Control Advisor software in the SIMCA-online program from Sartorius Stedim Biotech Data Analytics (formerly Umetrics) uses a patented imputation method based on partial least squares regression to provide that predictive ability for batch type systems. Using such an MPC set-up, it is possible to predict the future trajectory of a batch based on data from the current time point and change parameter set points to deliver required quality and performance.
Take, for example, the case of a 14-day cell culture process running in a bioreactor. It is possible to ascertain within the first couple days how the growth profile is likely to look over the remainder of the culture. This is because cells reveal their nature quickly, so it is possible to understand how they are growing and responding to the bioreactor environment right at the start of a run. With that information, a predictive model can calculate final conditions (e.g., titer and viable cell density) in the bioreactor. It then uses an iterative process to explore the effects of changing variables that can be easily manipulated (e.g., set points) until it identifies values for them to give the optimal outcome. Such a forecasting method allows for process deviations to be diagnosed and detected before they occur so the system can make corrective interventions.
MPC systems are most effective when users “train” them with information-rich data. The ambr® 15 and 250 systems are ideal tools for generating cell culture data sets for MPC design. They enable rapid and straightforward generation of 16-run training data sets by running multiple cell cultures in parallel with design of experiments (DoE) methodologies to introduce structured variation. The ease with which those systems can be scaled up to a commercial BIOSTAT® STR platform ensures that data generated at small scales will be relevant at large scale.
Conclusion
The biopharmaceutical industry is becoming increasingly confident in single-use bioprocessing technology, and this confidence allows it to implement such systems into the most critical applications within the commercial biomanufacturing environment. In the future, by combining data-driven analytics with mechanistic modeling, we will be able to improve significantly the “observability” of key quality parameters in single-use process platforms. We are seeing a real pull from the bioprocessing community to consider new ways of thinking about data and implement deep learning, machine learning, and artificial intelligence into Sartorius Stedim Biotech platforms. The future is exciting indeed. •
Chris McCready is lead data scientist at Sartorius Stedim Data Analytics.
You May Also Like





