- Sponsored Content
- Single Use
- Vaccines
Reacting to a Pandemic: Innovations in Vaccine DevelopmentReacting to a Pandemic: Innovations in Vaccine Development
February 5, 2021
Sponsored by Eppendorf
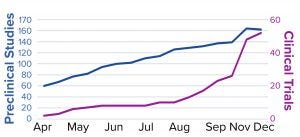
Figure 1: SARS-CoV-2 candidate vaccines in the preclinical and clinical evaluation phases
Traditionally, viruses for vaccines have been grown in embryonated hen eggs. But new challenges introduced by the COVID-19 pandemic have encouraged and catalyzed innovations in the field of vaccine development. The biopharmaceutical industry has recognized an advantage in mammalian cell-culture systems as promising alternatives to egg-based vaccine production. Cell lines can be cultured to large quantities in bioreactors, allowing for much shorter lead times, a more controlled production process, and a higher grade of reproducibility through standardization.
In this article, David Solbach explains how advancing single-use and automation technologies could accelerate cell-based vaccine development and production further. He also describes why stirred-tank bioreactors represent a key technology needed on the journey through developing and producing a new vaccine. Read on to learn how the flexibility and scalability of a well-established biopharmaceutical bioreactor cell-culture infrastructure could reduce costs and knowledge transfer required for vaccine development.
Fill out the form below to read the complete article now.
You May Also Like





