- Sponsored Content
Roivant Sciences Accelerates Process Development to Speed Orphan Therapy to MarketRoivant Sciences Accelerates Process Development to Speed Orphan Therapy to Market
November 22, 2017
Sponsored Content
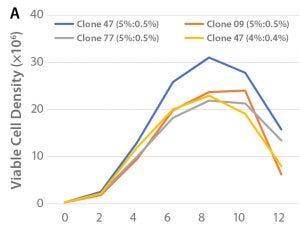
Figure 1: (A) Viable cell density over the culture period and (B) cell productivity on Day 12 of the tested clones in shakeflask cultures
Although the demographic of orphan therapies is small, making therapies for rare diseases available has a huge impact for the affected patients. Cooperation to expand capacity and expertise during process development and manufacturing for preclinical and clinical phase studies is one way to increase speed to market. This case study shares the work of GE’s Fast Trak Services team to help accelerate development of a process for cGMP production of material for toxicology studies. 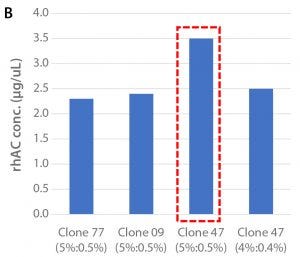 Frequent communication between the Fast Trak team and the client ensured transparency while protecting customer’s intellectual properties. GE scientists worked closely with Roivant Sciences to facilitate tech transfer, and a cGMP manufacturing process was developed. As a result, 400 grams of RVT-801 was produced for toxicology studies in 16 months.
Frequent communication between the Fast Trak team and the client ensured transparency while protecting customer’s intellectual properties. GE scientists worked closely with Roivant Sciences to facilitate tech transfer, and a cGMP manufacturing process was developed. As a result, 400 grams of RVT-801 was produced for toxicology studies in 16 months.
Background
Acid ceramidase is coded by the ASAH1 gene, of which two mutant copies lead to Farber disease. Roivant Sciences, a biotech company focusing on such rare conditions, has suggested recombinant human acid ceramidase (rhAC) as an enzyme replacement therapy. In preclinical models, cells take up rhAC, which thereafter breaks down ceramide stored in their lysosomes. The rhAC enzyme replacement therapy is intended to be a better option than bone-marrow transplant, which carries risk of toxicity with no guaranteed outcome.
To reduce risk in biomanufacturing and increase speed to market, Roivant Sciences initiated a collaboration with GE Healthcare’s Fast Trak Services to develop a process for production of rhAC. The goal of the project was to develop a cGMP production process that would provide sufficient material for toxicity studies. To improve manufacturability, a new process was developed from the original process, which had consisted of rhAC production in hollow-fiber bioreactor systems and purification in a three-step chromatography process using Con A Sepharose™ affinity, Blue Sepharose affinity, and Superose™ size-exclusion chromatography resins. The process at GE included upstream production in single-use Xcellerex™ stirred-tank bioreactor systems and downstream purification in three consecutive steps using modern Capto™ S ImpAct cation-exchange, Capto Butyl hydrophobic-interaction, and Capto Q anion-exchange chromatography resins.
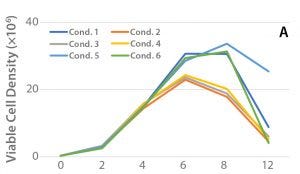
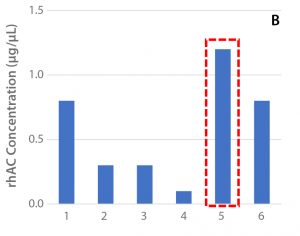
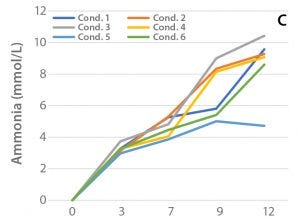
Figure 2: (A) Viable cell density over the culture period, (B) cell productivity on Day 12, and (C) ammonium levels for the selected clone (47) in shake-flask cultures
Selecting Best-Performing Clone
Clone selection was performed in 125-mL shakeflask cultures. Three different Chinese hamster ovary (CHO) cell clones (47, 09, 77) expressing rhAC were screened based on viable cell density and cell productivity, as determined by SDS-PAGE analysis. The cells were cultured in BalanCD™ CHO Grow A basal medium (Irvine Scientific) supplemented with 5.0% HyClone™ Cell Boost™ 7a and 0.5% Cell Boost 7b on a specified schedule. Culturing of the best performing clone (47) was repeated with feed ratio Cell Boost 7a to 7b of 4.0%/0.4%. Culturing was performed in 37 °C at an agitation rate of 120 rpm over 12 days. The results are shown in Figure 1.
 Optimizing Process Performance
Optimizing Process Performance
Optimization of feed conditions for the selected clone (47) was performed in 125-mL shake-flask cultures. Using BalanCD CHO Grow A as basal medium, the following fed-batch conditions were evaluated:
Condition 1 — once-daily bolus additions of 5.0% Cell Boost 7a and 0.5% Cell Boost 7b.
Condition 2 — once-daily bolus additions of 5.0% EfficientFeed™ B (Thermo Fisher Scientific)
Condition 3 — once-daily bolus additions of 5.0% Cell Boost 5.
Condition 4 — once-daily bolus additions of 5.0% Cell Boost 6.
Condition 5 — once-daily bolus additions of 5.0% Cell Boost 7a and 0.5% Cell Boost 7b with a temperature reduction on Day 4.
Condition 6 — once-daily bolus additions of 4.0% Cell Boost 7a and 0.4% Cell Boost 7b.
Culturing was performed in 37 °C (with a temperature shift on Day 4 for Condition 5) at an agitation rate of 120 rpm over 12 days. A sampling schedule was followed for analysis of viable cell density and cell productivity to support the decision on best-performing fed-batch condition. Ammonium levels were monitored over the culture period.
The results are shown in Figure 2. An outlying variable that separates Condition 5 from the other conditions is the temperature reduction on Day 4. Because of a slower metabolic rate, Condition 5 consumed less glutamine than the other conditions, and therefore showed lower ammonium concentrations, which might have contributed to the prolonged viable cell density of Condition 5. In addition to the improved viable cell density observed later in culture for Condition 5, the rhAC titer was significantly improved.

Table 1: Process conditions for optimization of bioreactor culture parameters
Best performance was achieved with Cell Boost 7a and 7b under Condition 5, with a peak viable cell density of 3 × 107 viable cells/mL (<2.5 × 107 viable cells/mL for all other conditions) and a productivity of ≥1.2 µg/µL (≤0.8 µg/µL for all other conditions).
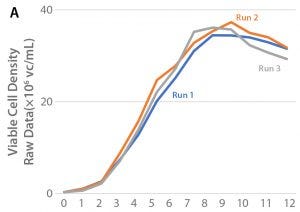
Figure 3: (A) Viable cell density and (B) cell productivity over the culture period for the selected clone (47) cultured in XDR10 bioreactor system
Process Scale-Up
For optimization of bioreactor culture parameters, the selected clone (47) was cultured in BalanCD CHO Grow A supplemented with once-daily bolus additions of 5.0% Cell Boost 7a and 0.5% Cell Boost 7b using the Xcellerex XDR-10 bioreactor system. Process conditions are listed in Table 1. Parameters monitored over the culture period were viable cell density, cell productivity, culture pH and partial CO2 pressure (pCO2), as well as concentrations of glucose, ammonium, and glutamine. Cell growth and productivity are shown in Figure 3.
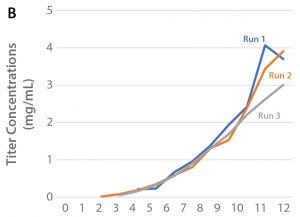 The best results were achieved with a 20-µm sparge configuration that provided a higher mass transfer (kLa) than the 0.5-mm drilled-hole configuration. As cells showed low sensitivity to shear forces, agitation could be increased at the end of the run to increase titers.
The best results were achieved with a 20-µm sparge configuration that provided a higher mass transfer (kLa) than the 0.5-mm drilled-hole configuration. As cells showed low sensitivity to shear forces, agitation could be increased at the end of the run to increase titers.
Small-Scale cGMP Production: Using bioreactor parameters from Run 3 in 10-L scale, the process was scaled to 200 L using the XDR-200 bioreactor system. To demonstrate equivalence between scales, the results were compared with the XDR-10 bioreactor run with regards to viable cell density, cell productivity, culture pH and pCO2, as well as concentrations of glucose, ammonium, and glutamine. Cell growth and productivity from two engineering runs (XDR-10 and XDR-200) and two cGMP runs (XDR-200) are shown in Figure 4.
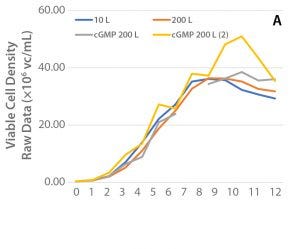
Figure 4: (A) Viable cell density and (B) cell productivity over the culture period for the selected clone (47) cultured in XDR10 and XDR-200 bioreactor systems
Downstream Purification and Final Formulation
To improve manufacturability, purity, and recovery, downstream purification of rhAC was optimized from the original academic laboratory-scale process based on Con A Sepharose affinity, Blue Sepharose affinity, and Superose size exclusion chromatography (1). It was also important that the developed downstream process was scalable and robust to allow for execution of a 200-L toxicology run. The optimized process comprises the following steps: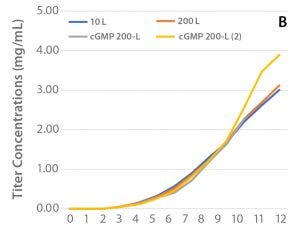
Harvest clarification by depth filtration.
Virus inactivation by low pH.
Capture using Capto S ImpAct cation exchange chromatography resin.
Intermediate polishing using Capto Butyl hydrophobic interaction chromatography resin.
Final polishing using Capto Q anion exchange chromatography resin.
Reduction of remaining virus particles by nanofiltration (20 nm).
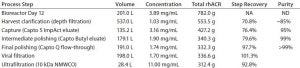
Table 2: Results from downstream purification of rhAC (NA = not applicable; ND = not detected)
The final product was concentrated by ultrafiltration through a filter with a Mr 10,000 nominal molecular weight cut-off (NMWC) to reach a target concentration in formulation agreed with the Roivant team. Results are shown in Table 2. Overall process recovery was ~44%, as determined spectrophotometrically at 280 nm, at a purity of >99%, as determined by SDS-PAGE analysis. Host cell DNA was reduced from initial 33 × 106 ng/L to <6.0 ng/L over the process.
Process Summary and Tech Transfer
To maximize rhAC production, the CHO cell clone that exhibited the highest cell growth and productivity was selected and upstream culture conditions were optimized. The optimized upstream process was successfully scaled from 125-mL shake flasks to 10-L and 200-L bioreactor cultures. To improve manufacturability, purity, and recovery of the downstream process, a purification process comprising clarification by depth filtration and purification in three consecutive chromatography steps was developed. Using this process, rhAC was produced in a 200-L process according to cGMP, generating sufficient material for toxicology studies. After completion of the project, a final report was delivered in accordance with set time lines, and the documentation required for technology transfer was prepared by the Fast Trak Services team.
Conclusions
Enzyme replacement using rhAC is an orphan therapy that was designated the need to get to market fast. This case study demonstrates development and optimization of a process for the production of rhAC, as a collaboration between Roivant Sciences and GE Healthcare’s Fast Trak Services team to ease risk and cost burdens, while increasing speed to market. Through clone selection as well as optimization of upstream production and downstream purification processes, sufficient amount of product could be produced in accordance with cGMP to be used in toxicology studies. Because technology transfer can be a challenge, Fast Trak scientists worked in close collaboration with the Roivant team to facilitate this process.
Acknowledgment
We thank Roivant Sciences for kindly providing us with permission for use of this body of work as a demonstration of our Fast Trak Services.
”Roivant greatly appreciates the way the GE team took this project on as if RVT-801 was their own program. They recognized the need for Farber’s patients and have gotten us in a position where we are successfully executing toxicology studies and are getting ready to begin clinical trials. Everyone has been focused on the patients and that is what made this relationship work so well.”
—Alex Tracy (Vice President of Pharmaceutical Development, Roivant Sciences)
Reference
1 He X, et all. Purification and Characterization of Recombinant, Human Acid Ceramidase. JBC 278, 2003: 32978–32986.
You May Also Like





