- Sponsored Content
- Chromatography
- Separation/Purification
Simple and Effective Method for Purification of DMT-On Oligonucleotides Using HIC ResinsSimple and Effective Method for Purification of DMT-On Oligonucleotides Using HIC Resins
Sponsored by Tosoh Bioscience
Within the biopharmaceutical industry, oligonucleotide drug pipelines have increased significantly because of the effectiveness of such drug products in treating devastating diseases. Downstream specialists need improved purification techniques for such highly valuable materials.
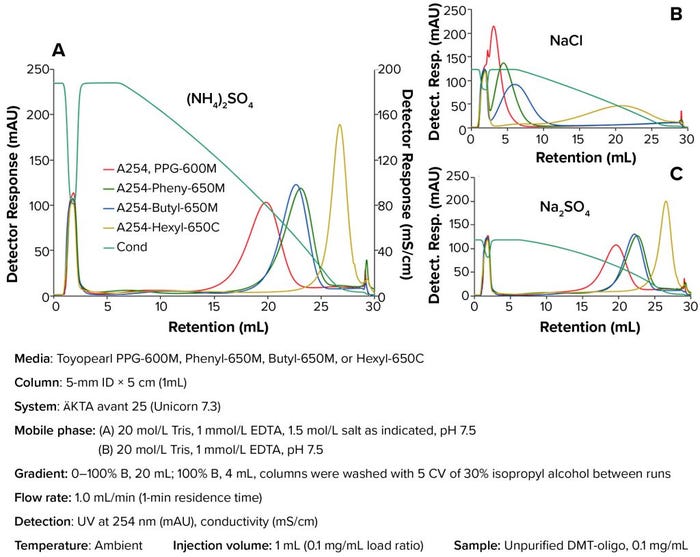
Figure 1: Impact of different salt conditions on hydrophobic-interaction chromatography (HIC) oligonucleotide purification
Dimethoxytrityl (DMT) is used to synthesize oligonucleotides and temporarily mask the characteristic chemistry of the 5ʹ-hydroxy functional group. DMT can be left on an oligonucleotide following synthesis to provide stability to a molecule during subsequent processing.
Herein, we describe a novel, effective, and high-recovery method for purifying a DMT-on oligonucleotide and effectively removing a DMT group from an oligonucleotide in a single purification step. This purification can be achieved by using hydrophobic- interaction chromatography (HIC) because the DMT-on group is strongly hydrophobic.
Materials and Methods
Oligonucleotide: 5ʹ-GAA TTC ATC GGT TCA GAG AC-3ʹ, a single-stranded DNA oligonucleotide 20-mer in length with a molecular weight of 6.141 kDa, was supplied at ~55% purity from TriLink.
Salts: Three different salts purchased from EMD Millipore were used in this study: sodium chloride (NaCl), sodium sulfate (Na2SO4), and ammonium sulfate (NH4)2SO4.
HIC Resins: Four Toyopearl HIC resins were selected for the study: PPG-600M, Phenyl-650M, Butyl-650M, and Hexyl-650C.
Conditions: See Figures 1–3.
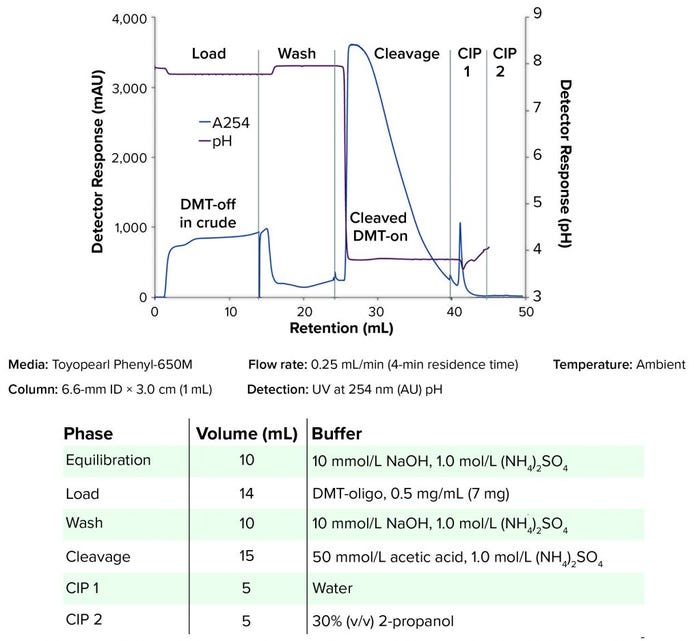
Figure 2: Cleavage of DMT-group directly on column; CIP = clean in place
Results and Discussion
Impact of Different Salt Conditions on Purification: Before the resin screening process, we determined the salt-tolerance limit of the oligonucleotide. Different concentrations of salts were added to the sample and tested to verify the concentration at which precipitation occurs: 0.5 mol/L, 1.0 mol/L, and 1.5 mol/L. At 1.5 mol/L salt concentration, the oligonucleotide was bound strongly to all HIC resins without sample precipitation. Thus, a 1.5-mol/L salt concentration was selected for use in subsequent studies.
Figures 1a and Figure 1c show that (NH4)2SO4 and Na2SO4 generated similar oligonucleotide peaks. In fact, all HIC resins gave similar elution profiles, and all selected resins effectively separated a DMT-off (flow-through peak) from a DMT-on oligonucleotide. The data also showed that Toyopearl Phenyl-650M and Butyl‑650M resins bound the oligonucleotide stronger compared with the Toyopearl PPG-600M resin. Toyopearl Hexyl-650C resin had the strongest binding. NaCl (Figure 1b) did not provide strong binding for the oligonucleotide on the resin, so the oligonucleotide eluted early at the beginning of the decrease of the salt gradient.
Sulfates of sodium and ammonium are most effective at promoting ligand–oligonucleotide interactions and are known to have a low destructive effect on sample structure. Ammonium sulfate demonstrated a more linear conductivity response when used in a gradient (compare Figures 1a and 1c) and thus was chosen for these studies.
One-Step Removal of DMT Protection Group: For the on-column removal of the DMT group from the oligonucleotide, Toyopearl Phenyl-650M resin was chosen as a representative of the screened Toyopearl HIC resins. Figure 2 shows that the DMT group from the oligonucleotide was removed (cleaved) directly on the column. DMT was cleaved by acidification at about pH 4 and subsequent elution of the DMT-off oligonucleotide. The DMT group was removed from the stationary phase during cleaning-in-place (CIP) of the column.
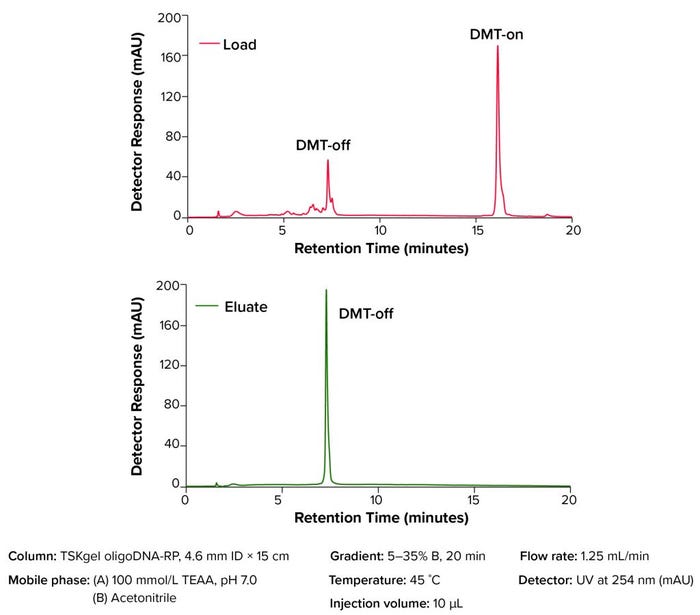
Figure 3: Analytical analysis of oligonucleotide from on-column cleaved DMT-group
Figure 3 shows the analysis of a crude oligonucleotide sample and the on-column cleaved DMT-off oligonucleotide fraction from Figure 2 using a TSKgel OligoDNA-RP high-performance liquid chromatography column. The DMT group was removed effectively from the oligonucleotide, and that on-column DMT cleavage resulted in a >99% pure DMT-off oligonucleotide at 99% recovery.
Effective Separation
Toyopearl HIC resins are effective in separating DMT-on and DMT-off oligonucleotides. High purity and recovery were achieved with an on-column cleavage procedure at low pH using Toyopearl Phenyl-650M.
William “Bill” Evans is an application scientist, and Phu Duong is a product manager of process media at Tosoh Bioscience LLC, 3604 Horizon Drive #100, King of Prussia, PA 19406; 1-484- 805-1219. Romain Dabre is senior product manager at Tosoh Bioscience GmbH (Griesheim, Germany). Toyopearl, TSKgel, and Tosoh Bioscience are registered trademarks of Tosoh Corporation.
You May Also Like






