- Sponsored Content
- Information Technology
- PAT
Simplifying the Bioprocessing 4.0 JourneySimplifying the Bioprocessing 4.0 Journey
Sponsored by Sartorius
 Bioprocessing 4.0, the biopharmaceutical version of Industry 4.0, is on course to become a reality in the next decade (1). This is because its “cyber-physical systems” that comprise cloud computing, connected systems, and digital process control offer many benefits. They include better process monitoring and management of a biologic’s critical quality attributes (CQAs) and the chance to control intensified processes for faster, less-expensive production of protein-based biologics and vaccines. Automation also can reduce the number of skilled operators needed while making manual tasks less error-prone and more efficient. That frees scientists to work on multiple processes simultaneously and respond rapidly to process issues, even from remote locations.
Bioprocessing 4.0, the biopharmaceutical version of Industry 4.0, is on course to become a reality in the next decade (1). This is because its “cyber-physical systems” that comprise cloud computing, connected systems, and digital process control offer many benefits. They include better process monitoring and management of a biologic’s critical quality attributes (CQAs) and the chance to control intensified processes for faster, less-expensive production of protein-based biologics and vaccines. Automation also can reduce the number of skilled operators needed while making manual tasks less error-prone and more efficient. That frees scientists to work on multiple processes simultaneously and respond rapidly to process issues, even from remote locations.
Bioprocessing 4.0 Roadblocks
For many biopharmaceutical companies, Bioprocessing 4.0 remains a bête noire that is insurmountable. Their perception is that the cost, resources, and time required to adopt best practices are just too much to justify without a guaranteed return on investment. Some people also believe that the concept of Industry 4.0 is difficult to adapt to biopharmaceutical manufacturing and is better suited to implementation in other industries. Such perceptions are due in part to a lack of awareness about the benefits that digital technologies such as simulation/data connectivity and the use of digital twins can bring to a biologics manufacturing environment. Additionally, insufficient connectivity across bioprocess equipment from different suppliers and gaps in technology make end-to-end, fully autonomous bioprocessing seem frustratingly out of reach.
Initiatives to develop standard data interfaces — including the recent BioPhorum plug-and-play principles (2) — are helping to move the goal posts, and implementing single-use (SU) technology can support a Bioprocessing 4.0 approach. But implementation is not as simple as connecting and qualifying systems in a manufacturing plant.
Bottlenecks can occur if equipment is not easy to integrate with higher-level supervisory control and data acquisition (SCADA) and/or manufacturing execution systems (MES). Such issues lead many companies to think of Bioprocessing 4.0 as “nice to have” and something they will get around to only after they have solved existing problems within their manufacturing networks.
However, increasing adoption of process intensification is pressuring manufacturers to develop a roadmap for Bioprocess 4.0 that not only addresses the roadblocks mentioned above, but also enables real-time monitoring, prediction, control, and release of critical process parameters (CPPs) at the entire process level.
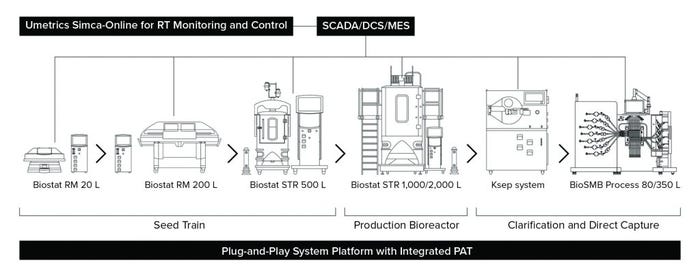
Figure 1: Representative setup of a highly automated process for intensified biomanufacturing (RT = real-time; PAT = process analytical technology)
An Incremental Framework for Integration
Being ready for Bioprocessing 4.0 does not require completion in one giant leap but, instead, should be a journey of manageable steps. Adopting available automation and real-time monitoring systems enabled by process analytical technology (PAT) from suppliers with a global network, deep process knowledge, and understanding of pain points can facilitate the transition toward Bioprocessing 4.0.
For example, BiosanaPharma, based in The Netherlands, is implementing this type of approach to help manufacture cost-effective biosimilars. The company is lowering the cost of goods in its manufacturing process by adopting end-to-end continuous manufacturing with its 3C process. The 3C platform (Figure 1) uses SU technology, PAT, and current good manufacturing practice (CGMP)- compliant plug-and-play systems from just a handful of suppliers to make the facility ready for Bioprocessing 4.0 (3). The simple pathway for implementation includes process automation designed for overarching data collection and feedback control between SCADA and controllers associated with SU systems. In upstream processing, the SU systems include a high–cell-density seed train with a 5-L Biostat STR bioreactor and production using 50-L dual SU Biostat STR bioreactors for alternating use, integrated with a tangential/alternating flow filtration (TFF/ATF) device followed by cell selection and/or harvest with Ksep SU centrifugation technology from Sartorius. The downstream process uses a BioSMB PD multicolumn chromatography (MCC) platform combined with flow-through polishing and intermediate filtration steps.
Ard Tijsterman, CEO at BiosanaPharma, explains: “The challenge in continuous processing is to be able to adapt the speed of the DSP to the output of the USP. Volume and product mass must be managed because all systems run simultaneously. To become Bioprocessing 4.0 ready requires a step-wise approach, which we have begun by integrating unit operation communication and control via SCADA and moving from off-line sampling to at-line and in-line analysis where practically possible. Working with fewer suppliers and using preintegrated systems makes plug and play easier as does software communication for flow control and data acquisition.”
PAT technology is helping operators implement real-time data gathering from bioreactors on CPPs, including pH, cell density, and viable cell count (VCC), to control and monitor BiosanaPharma’s media and feed strategies with minimal operator interactions. In the future, BiosanaPharma hopes to use Raman spectroscopy for analyte testing and quality by design (QbD). The company also wants to implement SIMCA MVDA tools to analyze, link, and contextualize the vast amounts of process data, which will help eliminate islands of information and enable operators to reap maximum benefits/efficiency from the data.
Commenting on the benefits of making a facility ready for Bioprocessing 4.0, Tijsterman adds, “Bringing down the cost of goods is a very strong driver for us. By using continuous processing, we can produce at high productivity and reduce our costs by 10-fold compared to the competition in biosimilar manufacturing. With a Bioprocessing 4.0 approach and by partnering with suppliers like Sartorius, who have a similar vision, we are confident that predicting upper and lower ranges of our CPPs will be possible in scale-up and commercial manufacturing. This approach will help to maintain CQAs of our monoclonal antibodies (MAbs) and validate our intensified processes, ensuring cost-effective, consistent product quality.”
Collaboration Is Key
Becoming ready for Bioprocessing 4.0 for current and next-generation intensified manufacturing seems to be a daunting prospect for smaller biopharmaceutical companies and for contract development and manufacturing organizations (CDMOs). However, as companies such as BiosanaPharma demonstrate, readiness is achievable by adopting a practical step-wise approach for which a logical, workable starting point is use of PAT, plug-and-play/industry-standard interfaces, data analytics, and improved controls for CPP monitoring and control. BiosanaPharma’s experience also shows that even the first step is a team sport in which partnering with experts such as Sartorius with experience and the right suite of products and capabilities can help. Tapping into a supplier’s expertise ensures that companies can address Bioprocessing 4.0 challenges to optimize a process while focusing on single-unit operations. This simplifies implementation of next-generation manufacturing enabled by process intensification for efficient production of high-quality, affordable biologics.
References
1 Demesmaeker M, et al. Bioprocessing 4.0 — Where Are We with Smart Manufacturing in 2020? Pharm. Outsourcing, September 2020; https://www.pharmoutsourcing.com/Featured-Articles/568001-Bioprocessing-4-0-Where-Are-We-with-Smart-Manufacturing-in-2020.
2 BioPhorum Plug and Play Workshop (2018). Accessed May 2021; https://www.biophorum.com/plug-and-play-workshop-how-to-make-standardized-interfaces-work-2.
3 Press release: BiosanaPharma Gets Approval to Start Phase 1 Clinical Trial for a Biosimilar Version of Omalizumab, the First Monoclonal Antibody Produced with a Fully Continuous Biomanufacturing Process; https://7301a529-be14-475b-8c71-b839f438e656.filesusr.com/ugd/3f142e_adbf792b6efa47ebb1aa1aa85770ca9c.pdf.
Corresponding author Ganesh Kumar is market entry strategy manager for protein based therapeutics; [email protected]. Michael Koch is head of sales, integrated solutions in the Asia–Pacific region; Artur Arsenio is head of product management for PAT, automation, and software; and Johannes Wagner is manager of systems engineering for BPS systems. All are with Sartorius in Germany.
You May Also Like





