- Sponsored Content
Single-Use Production Platforms for BiomanufacturingSingle-Use Production Platforms for Biomanufacturing
December 11, 2017
Sponsored Content
The pipeline of biopharmaceuticals remains strong, and the market for biologics could exceed US$450 billion by 2025. Analysts predict that sales within segments such as regenerative medicine and antibody–drug conjugates (ADCs) will grow faster than 20% each year. Yet considerable challenges remain for biopharmaceutical companies to overcome if they wish to be successful. First, they must reach the market quickly. Analysis by the Boston Consulting Group shows that the proportion of available value that a newly launched product can capture is a function of both the therapeutic advantage that it provides and its position in the launch of competing products. Being first to market is critical for commercial success of a new biologic.
Commercial risks inherent in developing new biological products have not gone away. Only a small proportion of such products that enter clinical assessment will make it through to a commercial launch. Although speed to market is important, companies must be careful to balance that need for speed against the risk that investments in process development activities will yield no return. It is a difficult balancing act. Cutting corners on development activities may allow companies to reach clinical phases quickly but also could result in them launching with poorly optimized production processes. Optimizing those processes after commercial launch is a very costly exercise because of regulatory obstacles that firms must overcome in making postapproval changes to manufacturing operations. When product sponsors enter the market with high operating costs, their profitability is reduced, making product sales vulnerable to attack from low-cost biosimilars. That is no longer a hypothetical situation: Regulators have been approving such products since 2013.
The implication is that senior executives from biopharmaceutical companies need to consider their approach to commercialization of biologics very carefully to reach the market quickly with efficient processes that deliver necessary product quality with as low a cost of goods (CoG) as possible. They should find ways to harness the skills and expertise that their suppliers have accumulated over many diverse projects from different clients. Biomanufacturers can feed that knowledge into their own activities in early, middle, and late development and augment their own resources with those of their supply partners. A critical factor when adopting such an approach is understanding how the range of available technologies and services can be brought together to achieve a product sponsor’s objectives successfully (Figure 1).
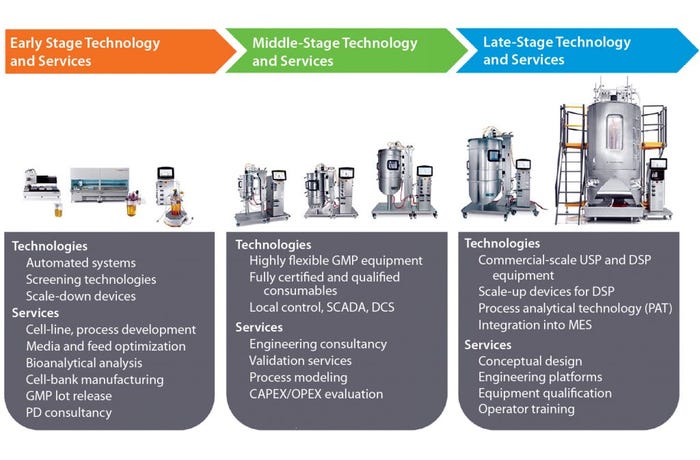
Figure 1: Needs within a standardized platform process
Biomanufacturers’ Needs Change During Development
During early stage process development, high-throughput screening technologies can accurately predict the performance of large-scale equipment and thus be used to rapidly develop and optimize bioprocesses. In some cases, automation can allow engineers to generate large amounts of process data and accelerate development time lines. Our process development consultants work with clients to identify work packages that make efficient use of laboratory-scale tools. They apply process platforms for antibodies, vaccines, and ADCs that we have put together based on many years of experience working with clients involved in those segments. Process platforms save considerable time by largely defining unit operations and their sequence in advance. More important, however, is that adopting platforms significantly increases a drug developer’s line of sight with detailed information on equipment requirements and likely costs of scaling up. By predicting future production needs, Sartorius Stedim Biotech process development consultants understand how to integrate critical services such as cell-line development and banking, media and feed development, and bioanalytical analysis into platform development to give customers a highly productive process in as little time as possible.
During middle-stage development, biopharmaceutical companies typically install flexible manufacturing capacity. Single-use technologies have been adopted widely for production of clinical supplies. The flexibility offered by such systems is invaluable to engineers fine-tuning production processes. Yet to prevent that flexibility from turning into costly complexity, biomanufacturers should configure their processes from predesigned consumables with robust supply chains. It is critical to ensure that technologies selected at this stage will be fully scalable to what a company anticipates its commercial scale of production to be. Sartorius Stedim Biotech single-use systems are scalable up to 2,000 L. Very often at that development stage, single-use systems will be controlled locally through SCADA systems.
As biomanufacturers prepare to launch their biologics onto the market, integrated production solutions become more important than the need for flexibility. Engineers must perform conceptual engineering designs and plan optimal layouts of production platforms within their facilities. Conceptual designs can be supported by process modeling programs such as BioSolve software from BioPharm Services. It helps biopharmaceutical companies optimize the likely capital and operating costs of a facility. Integration of processing equipment into manufacturing execution systems (MES) is more common during preparations for commercial manufacturing, requiring suppliers to have automation expertise. They can provide additional support through system qualification, operator training, and maintenance services.
Single-Use Platforms for Commercial Manufacturing
Sartorius Stedim Biotech believes that customers who implement its end-to-end process platforms benefit significantly from investments the supplier has made in ensuring biocompatibility, integrity, and assurance of supply for its single-use technologies. Its range of standardized consumables reduces supply chain complexity and improves quality assurance. Recently, the company has invested in a data-analytics platform that allows customers to make the best use of both laboratory and process-scale data, gain additional insight, and improve process control.
In April 2017, Nienke Vriezen of Synthon reported at ISPE Europe on her company’s implementation of a Sartorius Stedim Biotech single-use platform for production of monoclonal antibodies (MAbs) (Figure 2). The platform allows for production of different products, and its capacity is flexible depending on their production requirements. When product demand increases, Synthon can bring into operation two BIOSTAT® STR 2,000-L bioreactors. When smaller batches are required, the company can harvest from a BIOSTAT® STR 500-L bioreactor.
Vriezen described the full scalability of that Sartorius Stedim Biotech upstream platform from small-scale to the 2,000 L production scale. She also explained how much of her company’s downstream process requirements could be met from a single FlexAct® system from Sartorius Stedim Biotech. It allows Synthon to perform depth filtration, adjustment, nanofiltration, and ultrafiltration/diafiltration (UF/DF) steps during antibody purification.
Intensified Platforms of the Future
The biopharmaceutical industry must continue to develop production platforms that bring down CoG so medicines can reach patient populations around the world. Fed-batch processing in single-use systems are very costly when final production quantities of product exceed 1,000 kg/year. Traditional six-pack stainless steel facilities are a risky investment because companies must start their construction before evidence of the likely product demand can be understood fully. Such facilities require engineers to scale processes up to 12,000-L or even 15,000-L bioreactors — with unpredictable results.
The ultimate goal might be to move to fully continuous processing, but such technology has yet to be demonstrated fully especially at large scales. Sartorius Stedim Biotech is investing in upstream development tools such as ambr® systems and BIOSTAT® B-DCU benchtop bioreactors to help customers design continuous and intensified upstream processes. Those can be scaled up to the BIOSTAT® STR range of single-use bioreactors that allow for cell cultures of very high density through excellent mixing and mass-transfer characteristics. The Integrated Solutions team can support customers implementing high–cell-density cell banking, n – 1 perfusion systems, and concentrated fed-batch production. KSep single-use centrifuges are ideal for robust clarification of high-density cultures.
Conclusion
Challenges remain for biopharmaceutical companies bringing products to market. Product sponsors must manage uncertainty while accelerating biologics into the clinic and then onto market. Bioprocesses must meet stringent quality requirements with efficiency to provide the lowest possible overall costs. Sartorius Stedim Biotech is helping clients address those challenges through end-to-end single-use production platforms. Those platforms are underpinned by a robust single-use supply chain to ensure that customers always can meet their commitments to healthcare providers and patients. Sartorius Stedim Biotech has incorporated data analytics to enable clients to make sense of the process data they collect and use the resulting knowledge to improve quality and efficiency further. With these industry leading tools, we can help bring treatments to patients with unmet clinical needs and supply safe medicines around the world. •
Miriam Monge is director of marketing for integrated solutions at Sartorius Stedim Biotech.
You May Also Like





